Compound for determining content of metal ions in water environment, and its application
A metal ion, water environment technology, applied in the direction of analysis by chemical reaction of materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of lack of selectivity of zinc ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
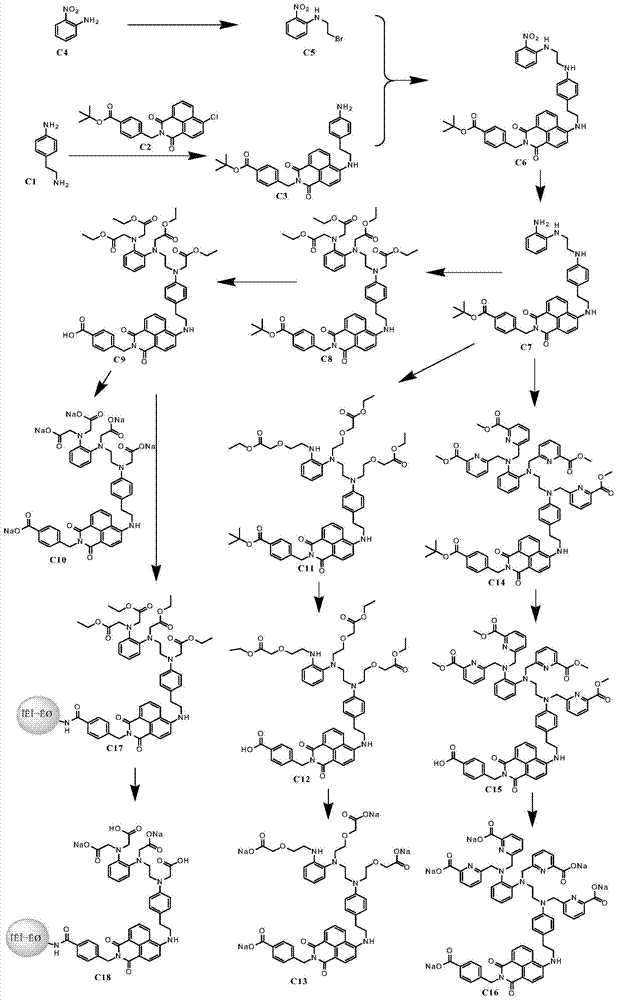
Embodiment 1
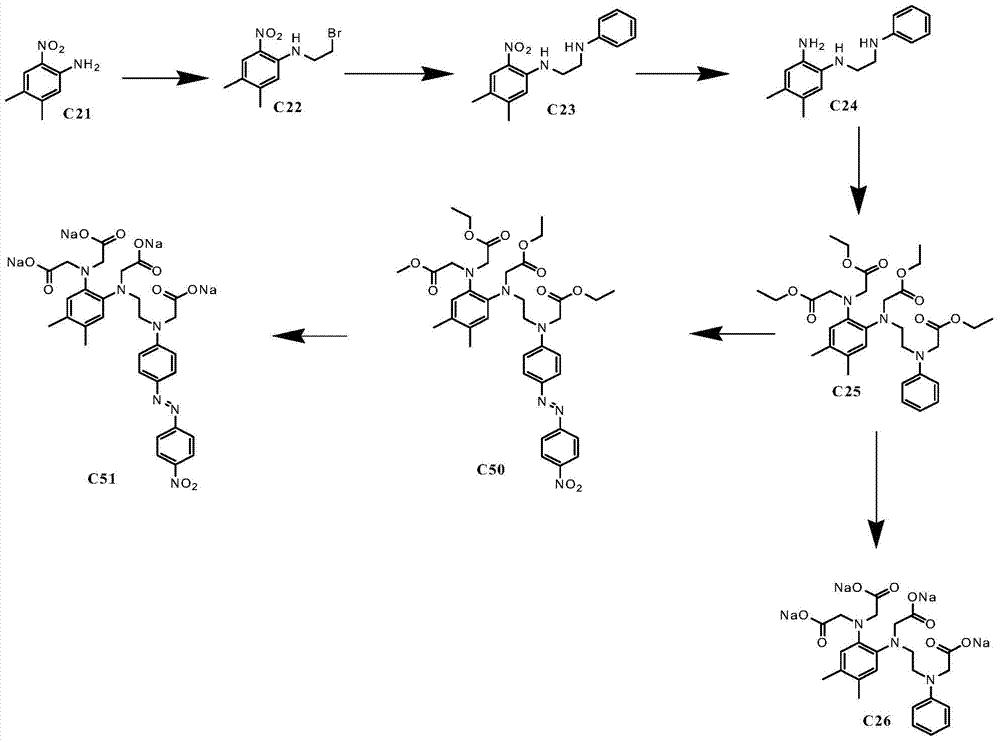
[0083] Embodiment 1: the synthesis of ion complexing group
[0084] Synthesis of compound C22: 2-nitro-4,5-dimethylaniline (C21) (29.9g0.18mol), 1,2-dibromoethane (235ml, 2.71mol), N,N-di Isopropylethylamine (DIEA, 90ml, 0.543mol) and 4-N, N-dimethylaminopyridine (DNAP, 3.10g, 0.025mol) were added to a three-necked flask, heated to 120°C, and kept warm for 18 After 1 hour, TLC detects that 2 / 3 of the raw materials have reacted, cooled, spin-dried the excess 1,2-dibromoethane, dissolved the solid in 100ml dichloromethane and 100ml water, washed the organic phase 3 times with water, and dried over anhydrous sodium sulfate , filtered, the solvent was spin-dried, the solid was purified through a column, and recrystallized from methanol to obtain 17 g of a yellow product (yield 30%). 1H NMR (CDCl3) δ=2.20(s, 3H), 2.25(s, 3H), 3.60(t, 2H), 3.78(t, 2H), 6.85(m, 1H), 7.25(m, 1H), 8.06 (s, 1H).
[0085]Synthesis of compound C23: C22 (6.8g0.025mol), aniline (2.31g, 0.025mol), potassi...
Embodiment 2
[0094] Synthesis of compound C50: 2 ml of p-nitroaniline (0.035 g, 0.27 mmol) was dissolved in tetrahydrofuran (THF) THF: water = 1:1 solution, sodium nitrite (0.017 g, 0.27 mmol was added, under ice-cooling, Add 0.05 ml of concentrated hydrochloric acid dropwise, stir in an ice bath for 1 hour, then add compound C25 (0.1 g, 0.18 mmol) in 2 ml of a solution of THF:water=1:1, stir in an ice bath for 2 hours, and turn to room temperature overnight. TLC detects that there is a new point to generate, a small amount of raw material has not reacted, add water, extract three times with EA, dry over anhydrous sodium sulfate, scraper, obtain orange-yellow product 0.06g (51%).1H NMR (CDCl ) δ=1.23( m, 12H), 2.26(m, 6H), 3.60(m, 4H), 4.00-4.20(m, 16H), 6.55-6.85(m, 5H), 7.15(m, 2H), 7.98(d, 2H) , 8.37(d, 2H).
[0095] Synthesis of compound C51: Dissolve compound C50 (0.06g, 0.08mmol) in methanol (2ml), add 0.1N sodium hydroxide (6ml), heat to 80°C, keep for 5min, cool, and stir overnigh...
Embodiment 3
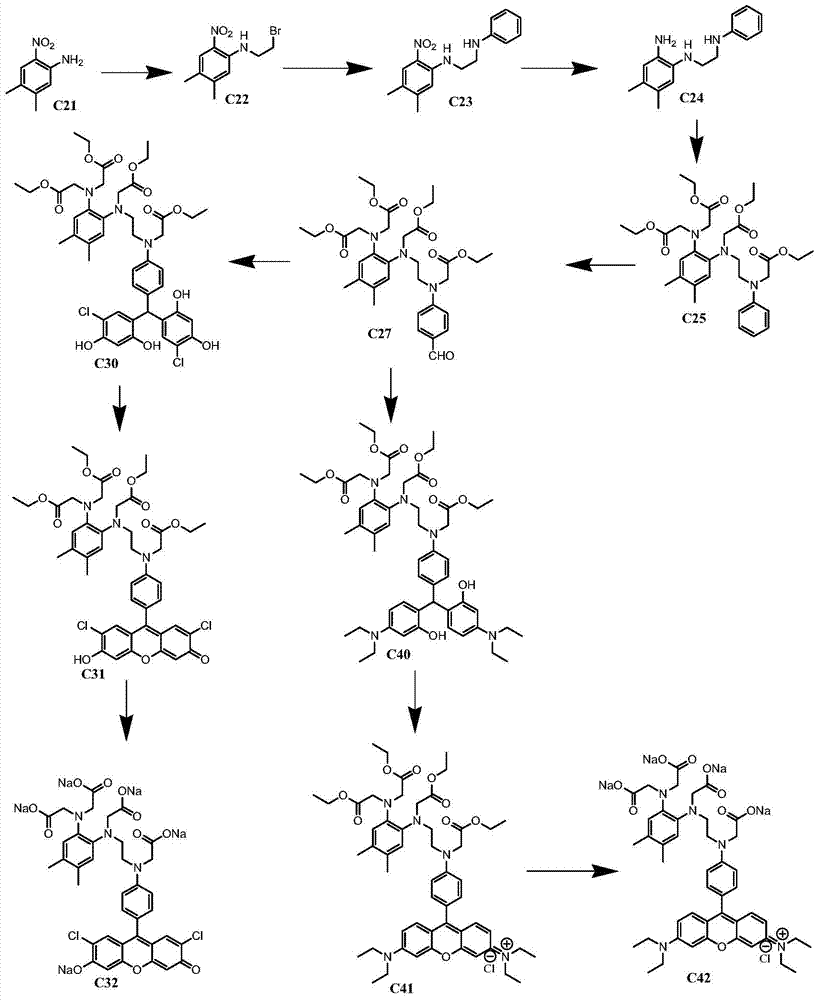
[0097] Example 3: Synthesis of compound C27: under ice bath, phosphorus oxychloride (7.7g, 0.05mol) was slowly added dropwise in DMF (8.0ml), stirred in ice bath for 0.5 hour, and then C25 (6.0g, 0.01 mol) of DMF (10ml) solution was added dropwise to the above solution, turned to room temperature and reacted overnight, TLC detected that the reaction was complete, poured into water, extracted with EA, dried, spin-dried, and passed through the column to obtain 3.0g of product (yield 48 %). 1H NMR (CDCl3) δ=1.23(m, 12H), 2.20(m, 6H), 3.55(m, 4H), 4.00-4.20(m, 16H), 6.60-6.85(m, 4H), 7.15(m, 2H), 9.80 (s, 1H).
[0098] Synthesis of compound C60: compound C27 (0.2g, 3.2*10 -4 mol) and 2-methyl-1-(3-sulfonylpropyl)naphtho[1,2-d]thiazolium hydroxide inner salt monohydrate (0.1g, 3.2*10 -4 mol) was added to the mixed solution of acetic acid (1.0ml) and acetic anhydride (1.0ml), reacted overnight at 100°C, the solution turned red, TLC detected that the reaction was complete, spin-dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com