Preparation method of 1,3-diene derivatives having aggregation-induced emission property
A technology of aggregation-induced luminescence and derivatives, which is used in the preparation of organic compounds, condensation of hydrocarbons with dehydrogenated hydrocarbons, and light-emitting materials. It can solve the problems of low efficiency and high cost, and achieve high reaction efficiency, improve efficiency, select high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
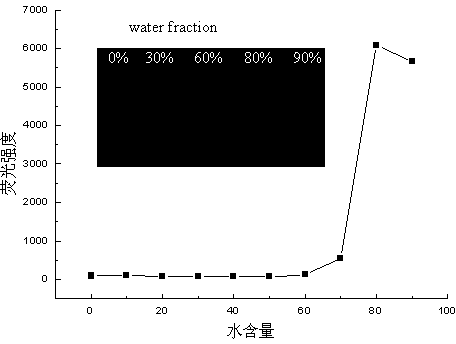
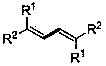
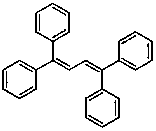
[0048] The preparation of 1,1,4,4-tetraphenyl-1,3-butadiene, the reaction formula is as follows:
[0049]
[0050] The preparation method is as follows:
[0051] (1) Add Pd(OAc) successively to the test tube 2 (12mg, 5mol%), N,N-dimethylformamide (2mL), benzyl chloride (126mg, 1.0equiv.), 1,1-diphenylethylene (180mg, 1.0mmol), and connected a balloon of one atmosphere of oxygen;
[0052] (2) Place the test tube containing the reaction mixture in an oil bath at 110°C and stir for 10 hours;
[0053] (3) After the completion of the reaction detected by TLC, cool to room temperature, add 10 mL of saturated brine to the reaction mixture for dilution, then extract with ethyl acetate (3×10 mL), combine the organic layers and wash with saturated brine, with anhydrous MgSO 4 Drying is carried out; the obtained organic phase is distilled off under reduced pressure to remove most of the solvent, and the target product can be obtained by preparative thin-layer chromatography.
[0...
Embodiment 2
[0056] The preparation of bromine-containing 1,1,4,4-tetrasubstituted-1,3-butadiene, the preparation method and the reaction formula are as follows:
[0057] (1) Synthesis of alkenes with the cross-coupling reaction of tosyl hydrazone and aryl or heteroaryl halide and the Wittig reaction, the reaction formula is as follows:
[0058]
[0059] (2) Using palladium to catalyze the self-oxidative dehydrogenation coupling reaction, the reaction formula is as follows:
[0060]
Embodiment 3
[0062] The bromine-containing 1,1,4,4-tetrasubstituted-1,3-butadiene prepared in Example 2 was prepared by Suzuki reaction to prepare aromatic group-containing 1,1,4,4-tetrasubstituted-1 ,3-butadiene derivatives.
[0063] Reaction formula:
[0064]
[0065] method:
[0066] (1) Add Pd(PPh 3 ) 4 (5mol%), K 2 CO 3 (2equiv), bromine-containing 1,1,4,4-tetrasubstituted-1,3-butadiene (1.0mmol), add toluene and water mixed solvent (volume ratio is 3:1), stir at room temperature for 30 minutes, Then add arylboronic acid (4.2 equiv);
[0067] (2) Place the test tube containing the reaction mixture in a 90°C oil bath and stir for 20 hours;
[0068] (3) After the completion of the reaction detected by TLC, cool to room temperature, add 10 mL of saturated brine to the reaction mixture for dilution, then extract with ethyl acetate (3×10 mL), combine the organic layers and wash with saturated brine, with anhydrous MgSO 4 Drying is carried out; the obtained organic phase is dist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com