Method for recovering and mechanically applying L-(+)-tartrate in D-ethyl ester production
A technology of tartaric acid and calcium tartrate, applied in the field of chemistry, can solve the problems of L-(+)-tartaric acid product quality failing to meet the requirements of separation, failing to meet quality standards, unqualified quality, etc., achieving easy control and improving quality , to avoid the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Production of L-(+)-Tartrate from L-(+)-Calcium Tartrate
[0042] In the 5000L calcium tartrate recovery kettle, add D-ethyl ester free mother liquor or L-ethyl ester free mother liquor into the recovery kettle, inject 70~80% liquid level, start stirring; add high-purity calcium chloride solid in batches, and react Generate L-(+)-calcium tartrate crystals, cool to 10~25°C to crystallize, centrifuge, wash, and spin dry to obtain L-(+)-calcium tartrate.
[0043] In a 5000L glass-lined reaction kettle, add 2000~3500kg of organic solvent acetone, stir, cool down to ≤10℃, add about 500~1000kg of L-(+)-calcium tartrate solid, cool down, and control the temperature in the kettle to ≤10℃ , slowly add 98% H2SO4 dropwise at 0°C~25°C, and stir at 10~30°C for 1~2hr after the dropwise addition. Suction filtration to remove impurities such as insoluble inorganic salt calcium sulfate, ammonium salt, sodium salt and calcium chloride complexes, and collect the acetone mother liquor o...
Embodiment 2
[0048] Production of L-(+)-Tartrate from L-(+)-Calcium Tartrate
[0049] In a 5000L glass-lined reaction kettle, add 2000~3500kg of organic solvent cyclohexane, freeze and cool, stir, add about 500~1000kg of L-(+)-calcium tartrate solid, stir, cool down, and control the temperature in the kettle to ≤10℃ , slowly add 98% H2SO4 dropwise at 0°C-25°C, and stir at 10-25°C for 1 hr after the dropwise addition. Suction filtration removes impurities such as insoluble inorganic salt calcium sulfate, and collects the cyclohexane mother liquor of L-(+)-tartaric acid to a storage tank, or directly transfers it to a concentration kettle.
[0050] Put the L-(+)-tartrate acetone mother liquor into a 5O00L glass-lined concentration kettle, inject the liquid level to about 70~85%, distill and recover the solvent under normal pressure or reduced pressure, and control the final vacuum degree -0.085~-0.098Mpa, T≤60°C, the volume of the filtrate is concentrated to 1 / 3, the material is viscous, ...
Embodiment 3
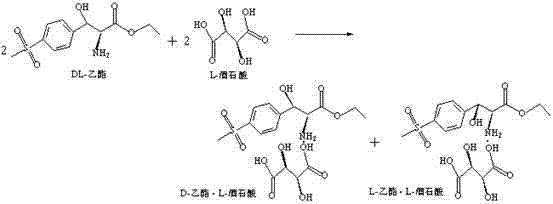
[0055] Recovery of L-(+)-tartaric acid for application and separation
[0056] Add 2200~3000kg of anhydrous methanol into a 5000L split kettle, add 220Kg of L-(+)-tartaric acid recovered by the above process, heat up, and keep warm at 25~35°C for 30min~60min, until the L-(+)-tartaric acid is completely Dissolve and set aside; add the prepared 400Kg DL-(±)-p-thymphenylphenylserine ethyl ester methanol solution into the split kettle, stir for 40-60 minutes, heat up to 45-66°C and reflux, and keep warm for 30-60 minutes ;After the reaction is completed, cool down to 28-33°C and prepare for pressure filtration;
[0057] At the same time, preheat the diaphragm filter press to about 28-33°C and keep it warm. When the temperature of the split kettle is cooled to about 28-33°C, discharge the material to the filter press for filtration. After the discharge is completed, press, Drying with compressed nitrogen, unloading to obtain D-(+)-p-thymphenyl phenylserine ethyl ester L-(+)-tart...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com