2-methyl-9-acridine(p-methoxy benzamido)thiourea, preparation method and uses thereof
A technology of acridinium thiourea and methyl, which is applied in the field of 2-methyl-9-acridine thiourea and its preparation, can solve the problem that there are no literature reports on derivatives with dual anti-tumor activities, and achieve anti-cancer effects Good, strong anti-tumor activity, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
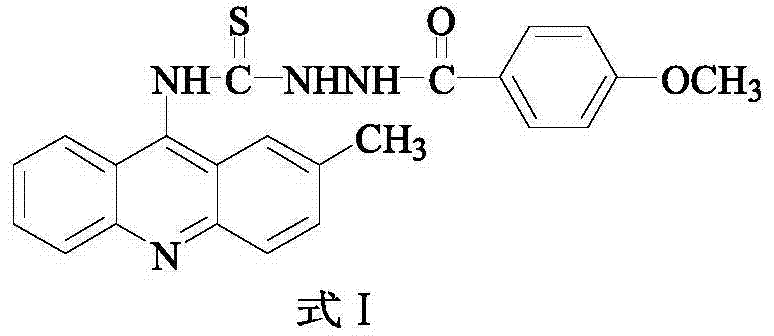
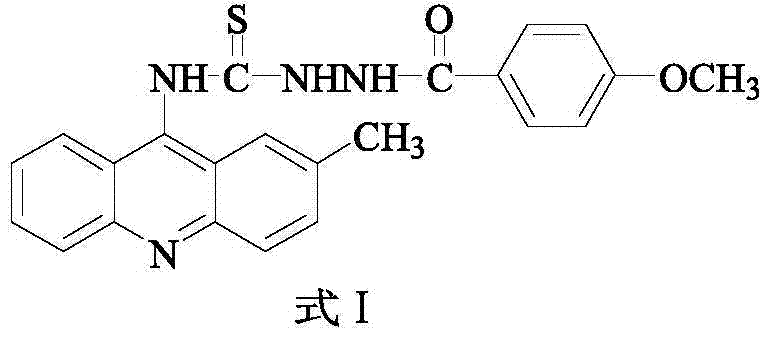
[0016] Example 1 Preparation of 2-methyl-9-acridine (p-methoxybenzamido) thiourea
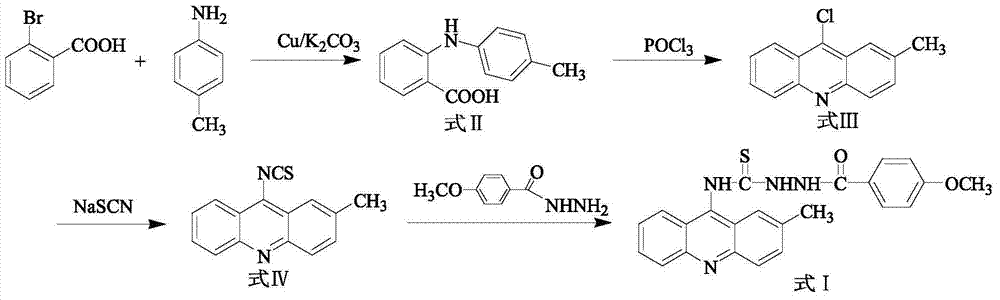
[0017] 1) In a 250mL three-necked flask, add 5.20g (26mmoL) of o-bromobenzoic acid, 3.64g (34mmoL) of p-methylaniline, 7.5g (36.2mmoL) of potassium carbonate and 0.3g (4.7mmoL) of copper powder, and then add 30 mL of isoamyl alcohol was used as a solvent, and stirred under reflux at 140° C. for 2 h. After the reaction, evaporate the solvent under reduced pressure, add 600 mL of water to the obtained residue, react at 80°C for 20 minutes, filter while hot, wash the filter cake, combine the water layer, acidify the water layer with concentrated hydrochloric acid to pH=2, and precipitate a large amount of light yellow Precipitate, filter with suction, and recrystallize the obtained solid with chloroform to obtain compound N-(p-methylphenyl)anthranilic acid (Formula II), with a yield of 77%;
[0018] 2) In a 100 mL round bottom flask, add the compound represented by formula II (18 moL) and 14.37 m...
Embodiment 2
[0021] Embodiment 2 Identification and analysis of compounds of the present invention
[0022] 2-methyl-9-acridine (p-methoxybenzamido) thiourea obtained by the above method 1 After H NMR nuclear magnetic resonance spectrum, FT-IR infrared spectrum, melting point and other tests, its chemical structure was confirmed by analysis.
[0023] The physical and chemical properties are as follows:
[0024] 1) Appearance: orange-yellow powder
[0025] 2) Melting point: 200~206℃
[0026] 3) Molecular weight: 416.50
[0027] 4) Molecular formula: C 23 h 20 N 4 o 2 S, the structural formula is as follows:
[0028]
[0029] 5) 1 H NMR nuclear magnetic resonance spectrum: the sample was dissolved in deuterated dimethyl sulfoxide (DMSO-d 6 ), measured at 400MHz, the obtained spectrum data are: δ: 11.26 (br, s, 1H, -NH), 10.41 (br, s, 1H, -NH), 10.22 (br, s, 1H, -NH), NH), 8.80(s, 2H, ArH), 8.05~8.15(m, 3H, ArH), 7.93(s, 2H, ArH), 7.77(t, 1H, ArH), 7.63(t, 1H, ArH), 8.15(d, 1H,...
Embodiment 3
[0031] Example 3 In Vitro Antitumor Activity Experiment
[0032] In vitro cytotoxicity assays were performed using the MTT method. The following four cell lines were used in the experiment: gastric cancer cell MGC-803, liver cancer cell BEL-7404, large cell lung cancer cell NCI-H460 and bladder cancer cell T24. 5-FU and cisplatin were used as reference substances. Data analysis Origin software was used for data processing.
[0033] experimental method:
[0034] 1) The selected cell lines were placed at 37°C, 5% CO 2 In an incubator under fully humidified conditions, inoculate and culture in PPMI1640 medium containing 10% inactivated newborn calf serum. Use an inverted microscope to observe the growth of the cells, replace the medium 2-3 times a week, passage once every 6-7 days, digest and passage with 0.25% trypsin when inoculating, usually take 3-4 passages, and use it for cells in the logarithmic growth phase in the experiment.
[0035] 2) Accurately weigh the sample ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com