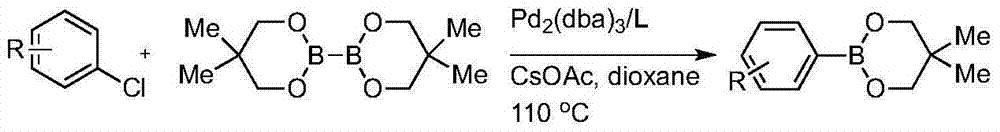Preparation method of high-sterically-hindered arylborate compound
An aryl boronate and compound technology, which is applied in the field of preparation of high sterically hindered aryl boronate compounds, can solve the problems of inapplicability of aryl chlorides and the like, and achieve the expansion of the scope of the substrate, the stability of the substrate, and the reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Synthesis of 2,6-dimethylphenylboronic acid neopentane glycol ester
[0018] In a 20 mL Schlenk tube, add tris(dibenzylideneacetone) dipalladium (0.0046 g, 0.005 mmol) and phosphine ligand (the ratio of palladium:phosphine ligand is 0.5mol%:4mol%), and then add poly Tetrafluoroethylene-coated magnetic stirring bar, the system was replaced by nitrogen protection, 0.5 mL of freshly distilled 1,4-dioxane was added, and stirred evenly for 10 minutes while adding to form a palladium complex. 2,6-Dimethylchlorobenzene (0.5 mmol), neopentyl glycol diboronate (0.6 mmol) and cesium acetate (1.5 mmol) were subsequently added under nitrogen. Finally 1,4-dioxane solution (1 mL) was added and stirring was continued for 5 minutes at room temperature. Then place the Schlenk tube in a preheated 110°C oil bath for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, and after the consumption of the aryl chloride was detected by thin ...
Embodiment 2
[0023] Example 2: Synthesis of 2,4,6-triethylphenylboronic acid neopentyl glycol ester
[0024] In a 20 mL Schlenk tube, add tris(dibenzylideneacetone) dipalladium (0.0046 g, 0.005 mmol) and ligand (palladium:ligand ratio is 1mol%:8mol%), and then add polytetrafluoroethylene Coated magnetic stirring bar, the system was replaced by nitrogen protection, 0.5mL of freshly distilled 1,4-dioxane was added, and stirred evenly for 10 minutes while adding to form a palladium complex. 2,4,6-Triethylchlorobenzene (0.5 mmol), neopentyl glycol diboronate (0.6 mmol) and cesium acetate (1.5 mmol) were then added under nitrogen. Finally 1,4-dioxane solution (1 mL) was added and stirring was continued for 5 minutes at room temperature. Then place the Schlenk tube in a preheated oil bath at 110°C for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, and after the consumption of the aryl chloride was detected by thin layer chromatography, the reactio...
Embodiment 3
[0027] Example 3: Synthesis of Neopentaneethylene Glycol 1,4-diboronate-2,3,5,6-tetramethylbenzene
[0028] In a 20mL Schlenk tube, add tris(dibenzylideneacetone)dipalladium (0.0092 g, 0.01 mmol) and ligand (palladium:ligand ratio is 1.0mol%:8.0mol%), and then add polytetrafluoroethylene A magnetic stirring bar coated with vinyl fluoride, the system was replaced by nitrogen protection, 0.5 mL of freshly distilled 1,4-dioxane was added, and stirred evenly for 10 minutes while adding to form a palladium complex. Then 1,4-dichloro-2,3,5,6-tetramethylbenzene (0.5 mmol), neopentyl glycol diboronate (1.2 mmol) and cesium acetate (3.0 mmol) were added under a nitrogen atmosphere . Finally 1,4-dioxane solution (1 mL) was added and stirring was continued for 5 minutes at room temperature. Then place the Schlenk tube in a preheated oil bath at 110°C for 24 hours. After the reaction was completed, the reaction tube was cooled to room temperature, and after the consumption of the aryl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com