Preparation method of low-melting-point regenerated copolyester
A technology of copolyester and low melting point, applied in the field of preparation of low melting point regenerated copolyester, can solve problems such as low catalytic efficiency of catalyst, and achieve the effect of good economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
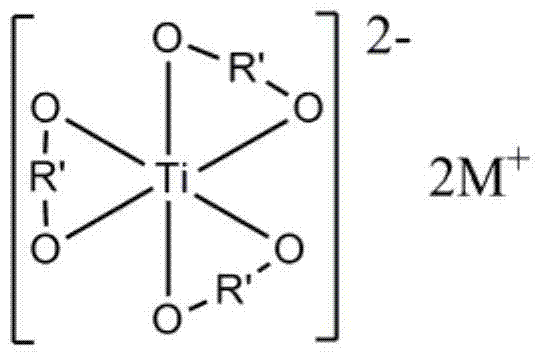
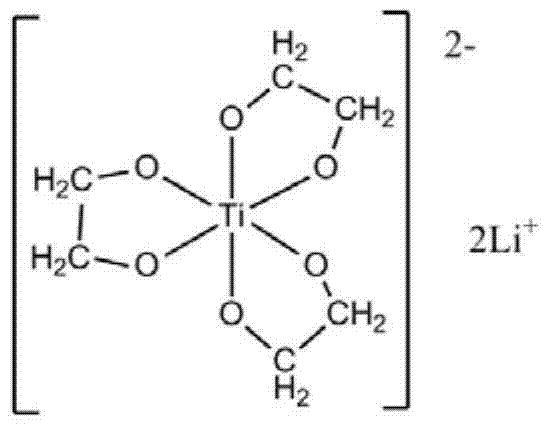
[0050] First, the preparation of lithium glycol titanate includes the following steps:
[0051] a) Mix tetraethyl titanate uniformly with ethylene glycol at 50°C under nitrogen protection, and the molar ratio of tetraethyl titanate to ethylene glycol is 1:100;
[0052] b) Add lithium hydroxide, the molar ratio of lithium hydroxide to tetraethyl titanate is 2.05:1, continue stirring until the reaction system is a uniform and transparent liquid, and then increase the reaction temperature to 180°C;
[0053] c) During the reaction process, reflux the ethylene glycol and remove the low-boiling ethanol and water produced by the reaction. The reaction time is 3 hours. After the reaction, the temperature is 190℃, the pressure of the reaction system is 0.5atm, and within 0.5min. Evaporate the ethylene glycol in the system completely, and evaporate the glycol in the system completely to obtain a white solid. This solid is recrystallized three times with ethanol-chloroform to obtain colorless c...
Embodiment 2
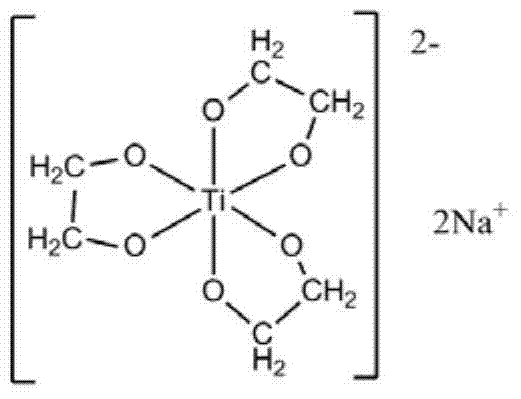
[0066] Firstly, the preparation of sodium glycol titanate includes the following steps:
[0067] a) Mix tetra-n-butyl titanate with ethylene glycol uniformly at 150°C under nitrogen protection, and the molar ratio of tetra-n-butyl titanate to ethylene glycol is 1:50;
[0068] b) Add sodium hydroxide, the molar ratio of sodium hydroxide to tetra-n-butyl titanate is 2.03:1, continue to stir until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 210°C;
[0069] c) During the reaction, reflux the ethylene glycol and remove the low-boiling n-butanol and water generated by the reaction. The reaction time is 2 hours. After the reaction is completed, the temperature is 210°C and the pressure of the reaction system is 0.8atm within 1min. Evaporate the ethylene glycol in the system completely, and evaporate the glycol in the system completely to obtain a white solid. This solid is recrystallized three times with ethanol-chloroform to obtain colorl...
Embodiment 3
[0082] Firstly, the preparation of potassium propylene glycol titanate includes the following steps:
[0083] a) Mix tetraisopropyl titanate with 1,2-propanediol uniformly at 100°C under nitrogen protection, and the molar ratio of tetraisopropyl titanate to 1,2-propanediol is 1:80;
[0084] b) Add potassium hydroxide, the molar ratio of potassium hydroxide to tetraisopropyl titanate is 2.02:1, continue to stir until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 200°C;
[0085] c) During the reaction, reflux 1,2-propanediol and remove the low-boiling isopropanol and water produced by the reaction. The reaction time is 4 hours. After the reaction, the temperature is 200°C and the reaction system pressure is 0.7atm for 1min. In the middle, the ethylene glycol in the system is completely evaporated, and the diol in the system is completely evaporated to obtain a light yellow solid. This solid is recrystallized three times with ethanol-chl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com