Recombinant long-acting human hyaluronidase, and encoding gene, production method and application thereof
A technology of human hyaluronidase and hyaluronidase, applied in the field of recombinant long-acting human hyaluronidase, can solve problems such as short half-life, and achieve the effects of prolonging the validity period, prolonging the half-life, and using safe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Objective: Gene construction, expression, immunological detection, activity detection and reactor production of fusion protein PH20-HSA or PH20-IgFc.
[0040] Method: Construct the structural gene PH20-HSA (SEQIDNo.6) or PH20-IgFc (SEQIDNo.7) into GC-rich animal cell expression vectors pMH3, 4, 5 (SEQIDNo.8, 9, 10) by PCR middle. After these several expression plasmids were prepared in small quantities, they were stably transfected into CHO cells cultured in serum-free suspension. Through G418 screening, stable clones were picked manually with a pipette tip and placed in a 96-well plate. When the confluence of the cells is greater than 50%, replace with fresh serum-free medium; after 3-6 hours, collect the medium samples, detect the expression level by anti-IgG2Fc antibody dot hybridization or ELISA method, and select multiple clones with the highest expression level, Continue to carry out serum-free culture and do passage stability research in small conical shake fla...
Embodiment 2
[0044] Objective: To separate and purify the fusion protein PH20-HSA or PH20-IgFc.
[0045] Method: The protein separation and purification strategy used in this example is (1) PH20-IgFc mainly adopts Protein A affinity purification; (2) PH20-HSA adopts the purification process of ion series hydrophobic blue gel.
[0046] (1) PH20-IgFc collection and purification.
[0047] After the culture medium was harvested, it was centrifuged at 5000 rpm for 6 minutes, and the centrifuged supernatant was taken for filtration. The pore sizes of the three filter membranes were 0.8 μm, 0.45 μm, and 0.22 μm, respectively. Load the sample to the Mabselect column that has been equilibrated with equilibrium solution A (20mM Tris+100mM NaCl, pH7.4), rinse until the baseline remains unchanged, and use eluent B (100mM Gly+50mM Arg+0.01%Tween80 , pH3.5) for elution. The separation and purification of the product was detected by SDS-PAGE.
[0048] (2) PH20-HSA collection and purification
[0049]...
Embodiment 3
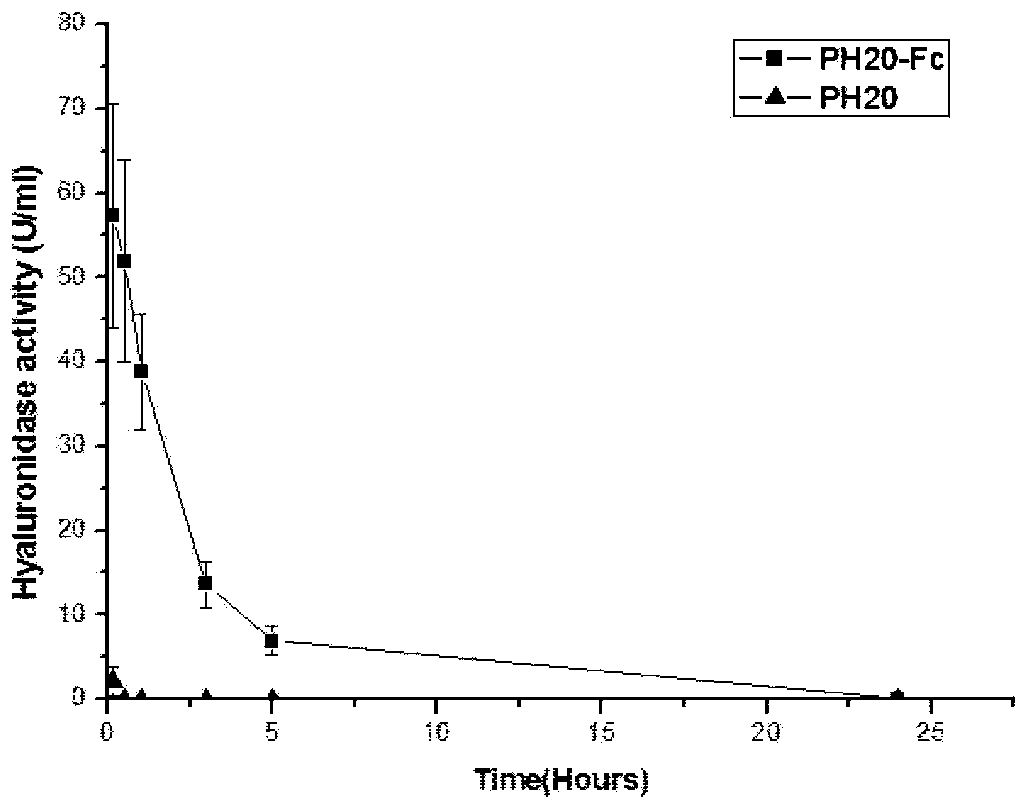
[0056] Objective: Study on preparation and in vitro and in vivo activities of fusion protein PH20-IgFc or PH20-HSA
[0057] Method: The formulation of hyaluronidase fusion protein PH20-IgFc injection is as follows:
[0058] PH20-IgFc+10mM Na2HPO4+0.03%CaCl2+0.1%EDTA-Na2+145mM NaCl+0.1%HSA, pH5.5-7.5
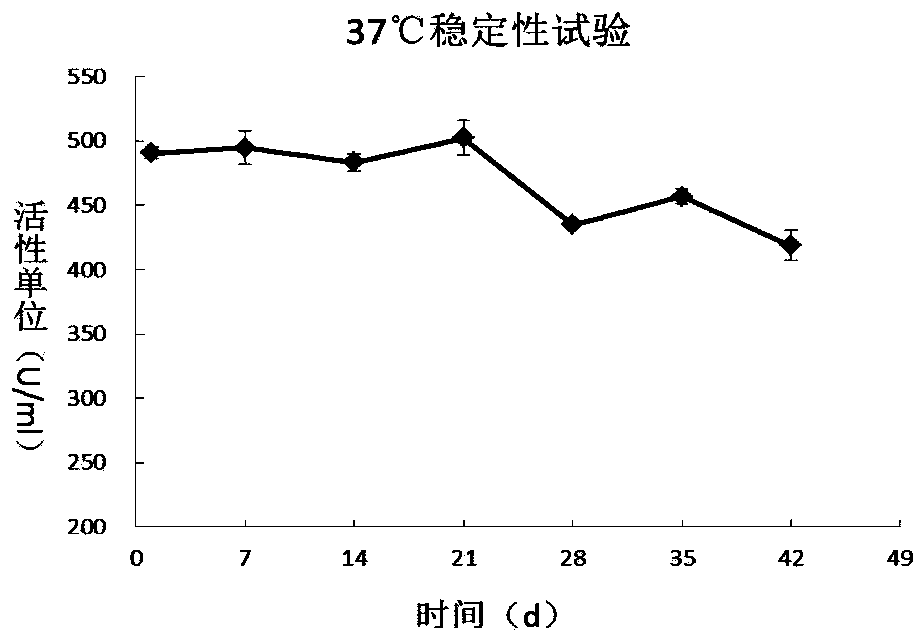
[0059] Dispense the 500U / ml PH20-IgFc filtered solution into 2ml ampoules, and place 25 bottles of the solution in an accelerated test incubator at 37°C with a humidity of 75±5%. Samples were taken on days 0, 7, 14, 21, 28, 35, and 42, respectively, according to the hyaluronidase activity test in the appendix of the 2010 edition of the Pharmacopoeia, and the results of the stability test (Table 1, figure 1 ) showed that from the 28th day, the activity of hyaluronidase decreased significantly (about 15%).
[0060] Table 1 PH20-IgFc stability test results at 37°C
[0061]
[0062]
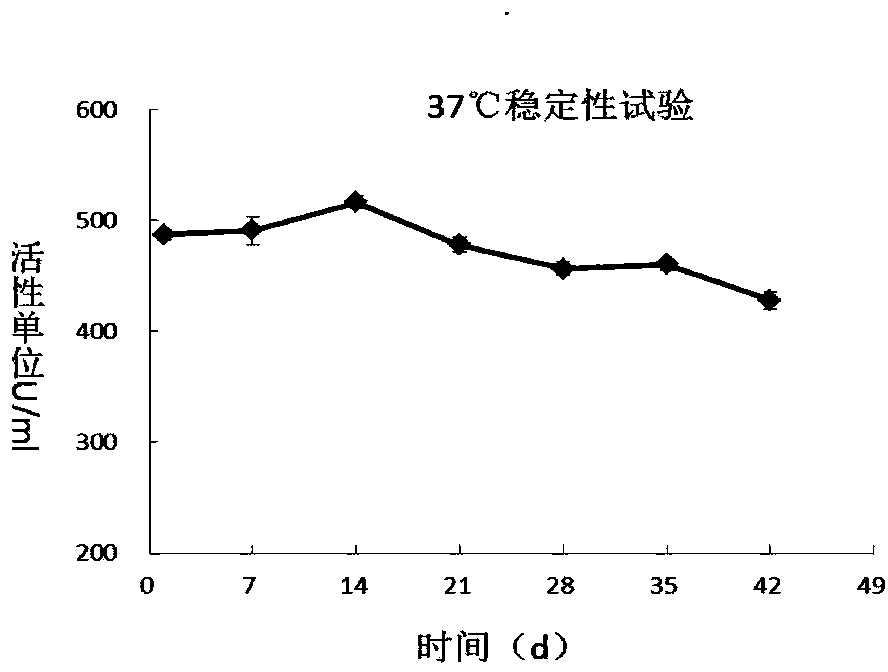
[0063] The formulation of the hyaluronidase fusion protein PH20-HSA injection is as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com