Nano-emulsion containing perillaldehyde, citral and nystatin and preparation method of nano-emulsion
A technology of nystatin and nanoemulsion, applied in antifungal agents, antibacterial drugs, pharmaceutical formulations, etc., can solve problems affecting bioavailability, achieve good skin permeability, and prolong metabolic time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
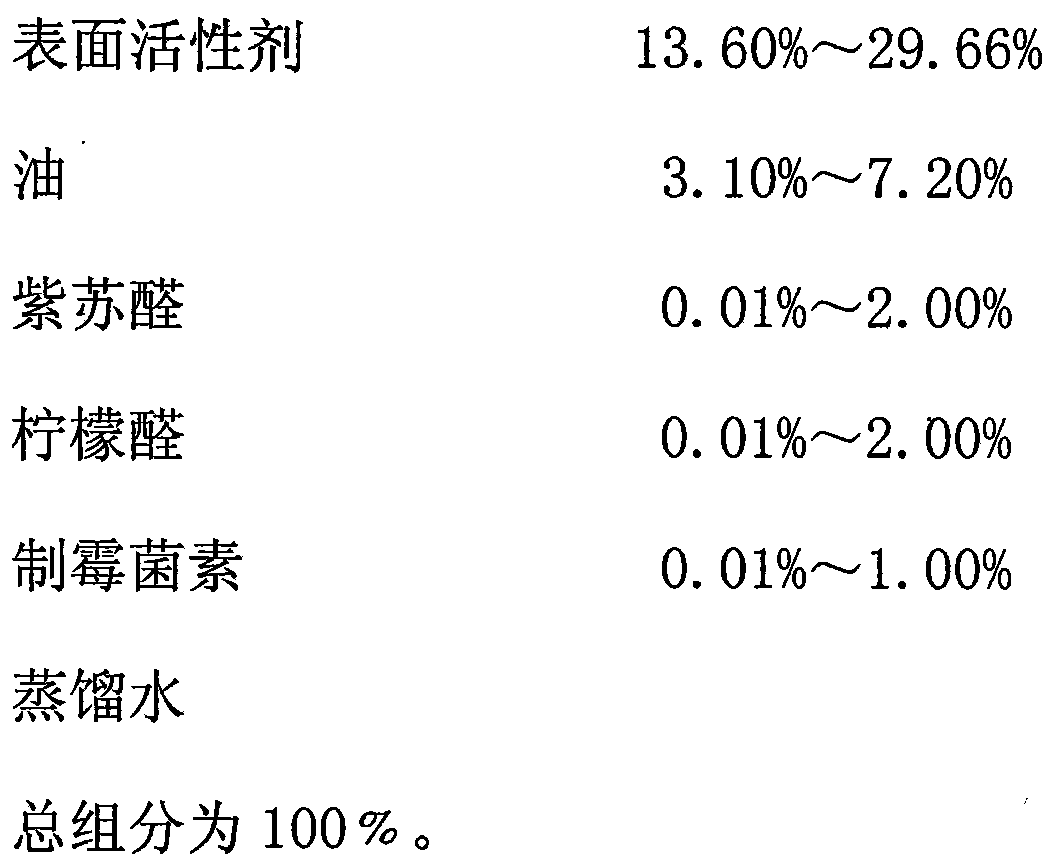
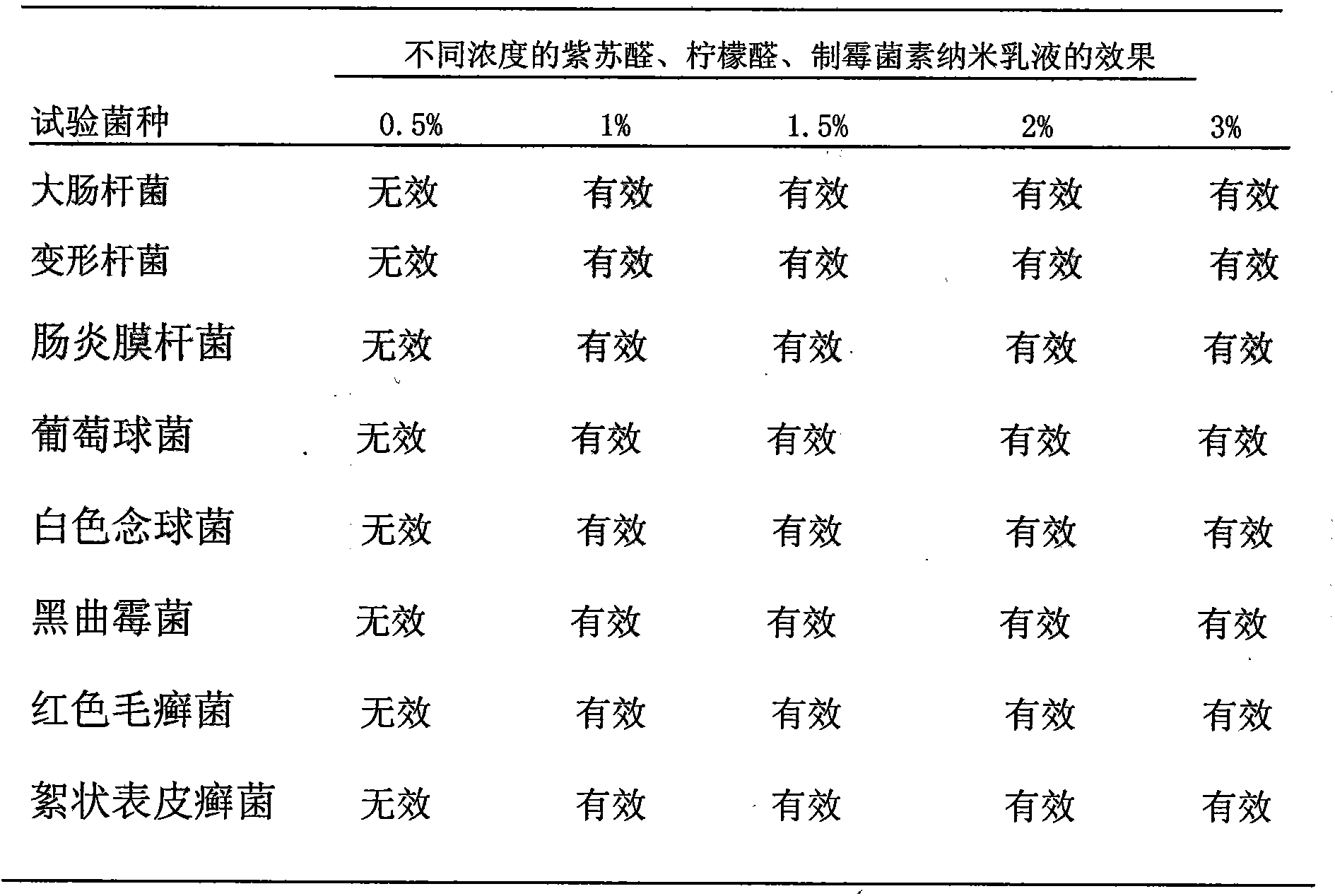
[0036] Accurately weigh 4.5g of hydrogenated castor oil polyoxyethylene ether, 1.0g of ethanol (in which 0.1g of perillaldehyde, 0.1g of citral, and 50IU of nystatin are dissolved), 0.5g of olive oil, and stir manually at room temperature of 20°C Mix it well, then slowly add distilled water to it, and stir manually while adding water. At the beginning, the viscosity of the system is small. As the amount of water increases, the system will become viscous. At this time, the system may appear liquid crystal or water-in-oil type Nanoemulsion, continue to drop and constantly stir, when system suddenly thins out, what produce now is O / W type colorless transparent perillaldehyde, citral, nystatin nanoemulsion, claim its weight as 10.0g.
Embodiment 2
[0038] Accurately weigh 4.5g of castor oil polyoxyethylene ether, 0.5g of ethanol (in which 0.05g of perillaldehyde, 0.05g of citral, and 60IU of nystatin are dissolved), 0.5g of isooctyl palmitate, and at room temperature of 22°C, Stir manually to mix it well, then slowly add distilled water to it, and stir manually while adding water. At the beginning, the viscosity of the system is small. As the amount of water increases, the system will become viscous. At this time, the system may appear liquid crystal or oil. Water-type nano-emulsion, continue to drop and stir continuously, when the system suddenly becomes thinner, what is produced at this time is the O / W type light yellow transparent perillaldehyde, citral, and nystatin nano-emulsion, which weighs 10.00 g.
Embodiment 3
[0040] Accurately weigh castor oil polyoxyethylene ether 3.0g, 1,3-butanediol 1.0g (wherein dissolve perillaldehyde 0.025g, citral 0.025g, nystatin 70IU), myristyl isopropyl ester 0.44g, in At room temperature of 23°C, stir it manually to mix it evenly, then slowly add distilled water to it, and stir manually while adding water. The viscosity of the system is small at the beginning. Liquid crystal state or water-in-oil nanoemulsion appears, continue to drop and stir continuously, when the system suddenly becomes thinner, the O / W type light yellow transparent perillaldehyde, citral, nystatin nanoemulsion will be produced , which weighs 10.0g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com