Magnetic ordered mesopore composite material with cavity structure, synthesis and application
A composite material and magnetic technology, applied in silicon compounds, radioactive purification, alkali metal compounds, etc., can solve the problem of less adsorption sites, and achieve the effect of large specific surface area and pore volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
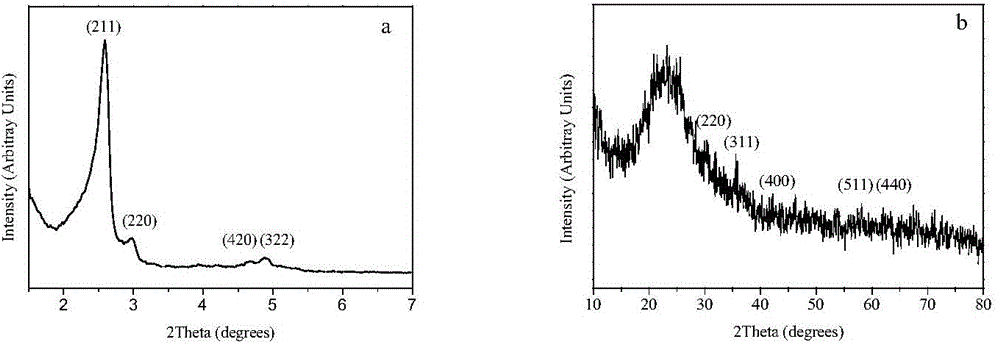
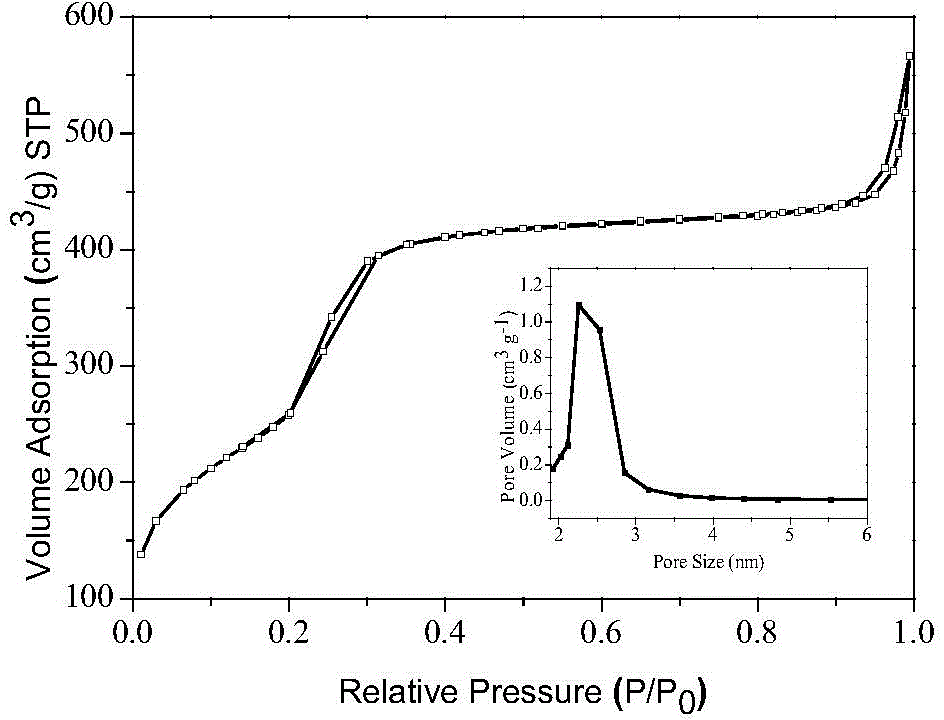
Image
Examples
Embodiment 1
[0033] Example 1: CoFe with cavity structure 2 o 4 Preparation of MCM-48 Magnetic Composite Material
[0034] (1) CoFe coated with oleic acid 2 o 4 Synthesis of magnetic nanoparticles: the configured 20mL2mol / L FeCl 3 solution and 20mL of 1mol / L CoCl 2 The solution was mixed and stirred for 30 minutes, then 4 mmol of oleic acid and 16 mL of 3.5 mol / L NaOH solution were added, and the temperature was raised to 80°C under high-speed stirring. Use HCl to adjust the pH of the solution to about 5. After the supernatant and the black precipitate in the lower layer appear, collect the black floc with a magnet, wash with deionized water and absolute ethanol for 3 times, and put it in a 60°C refrigerator. Dry in a vacuum desiccator for 24 hours.
[0035] (2) CTAB stabilized CoFe 2 o 4 Synthesis of magnetic nanoparticles: by the oil-water microemulsion method, take 0.12g of oleic acid-coated cobalt ferrite nanoparticles in 3mL of chloroform, after ultrasonication for 15min, take...
Embodiment 2
[0041] Example 2: The specific implementation steps of this embodiment differ from Example 1 in that acid is used to adjust the pH value of the stock solution after stirring to 9.54; others are the same as Example 1.
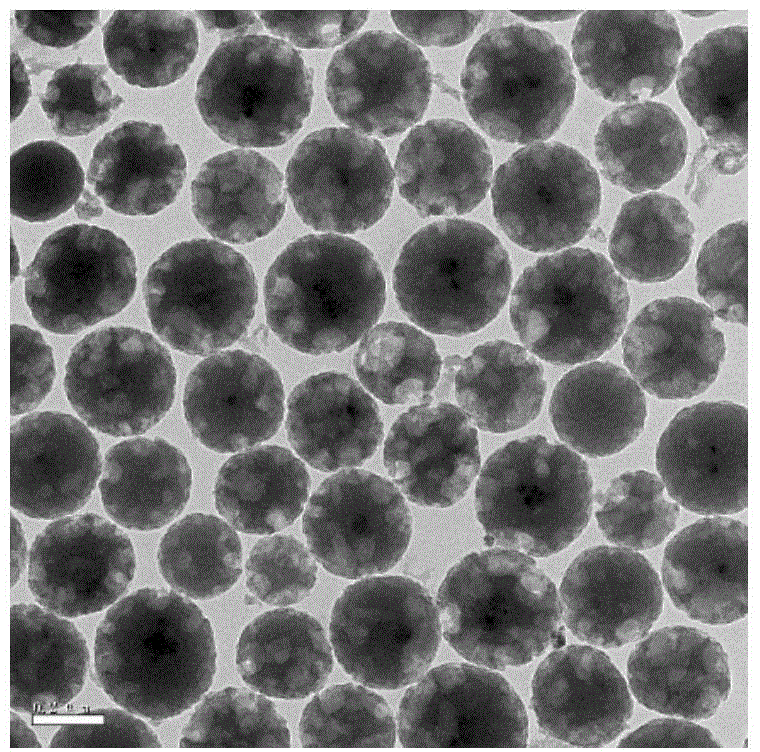
[0042] Figure 4 It is the TEM picture of this example. It can be clearly seen from the figure that the silica spheres coat the cobalt ferrite magnetic nanoparticles inside. The silica spheres are evenly distributed, with continuous edges, and the size is between 200 and 400nm. .
Embodiment 3
[0043] Example 3: The specific implementation steps of this embodiment differ from Example 1 in that acid is used to adjust the pH value of the stock solution after stirring to 10.0; others are the same as Example 1.
[0044] Figure 5 It is the TEM picture of this example. It can be clearly seen from the figure that the silica spheres are coated with cobalt ferrite magnetic nanoparticles inside. There are continuous and discontinuous edges of the silica spheres, and the size ranges from 160 to Between 360nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com