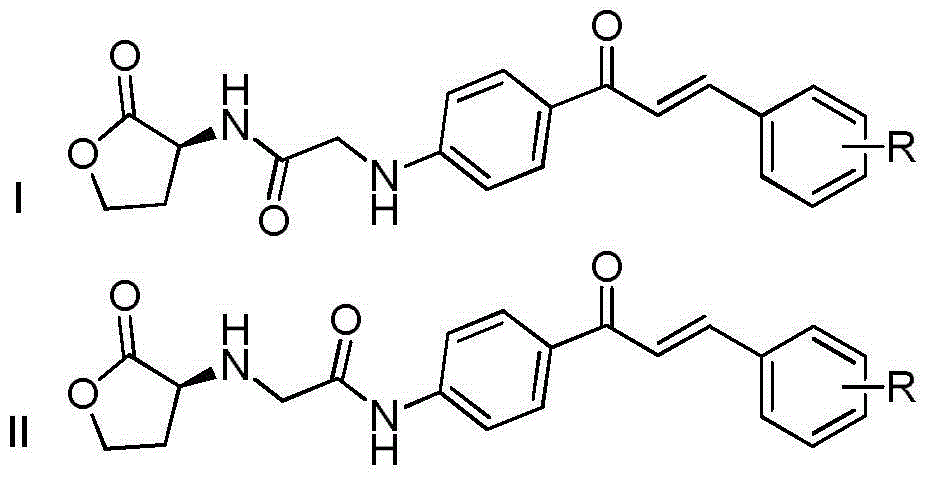
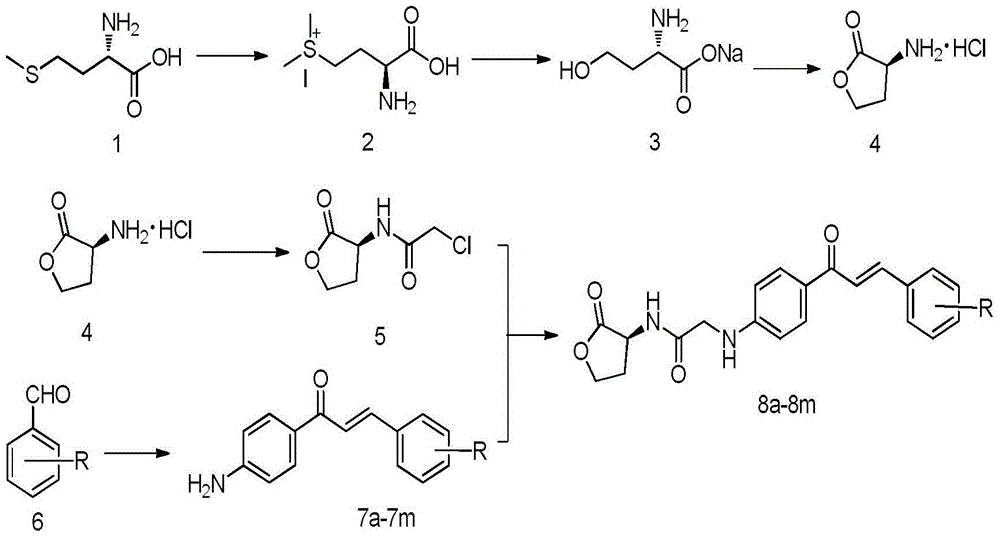
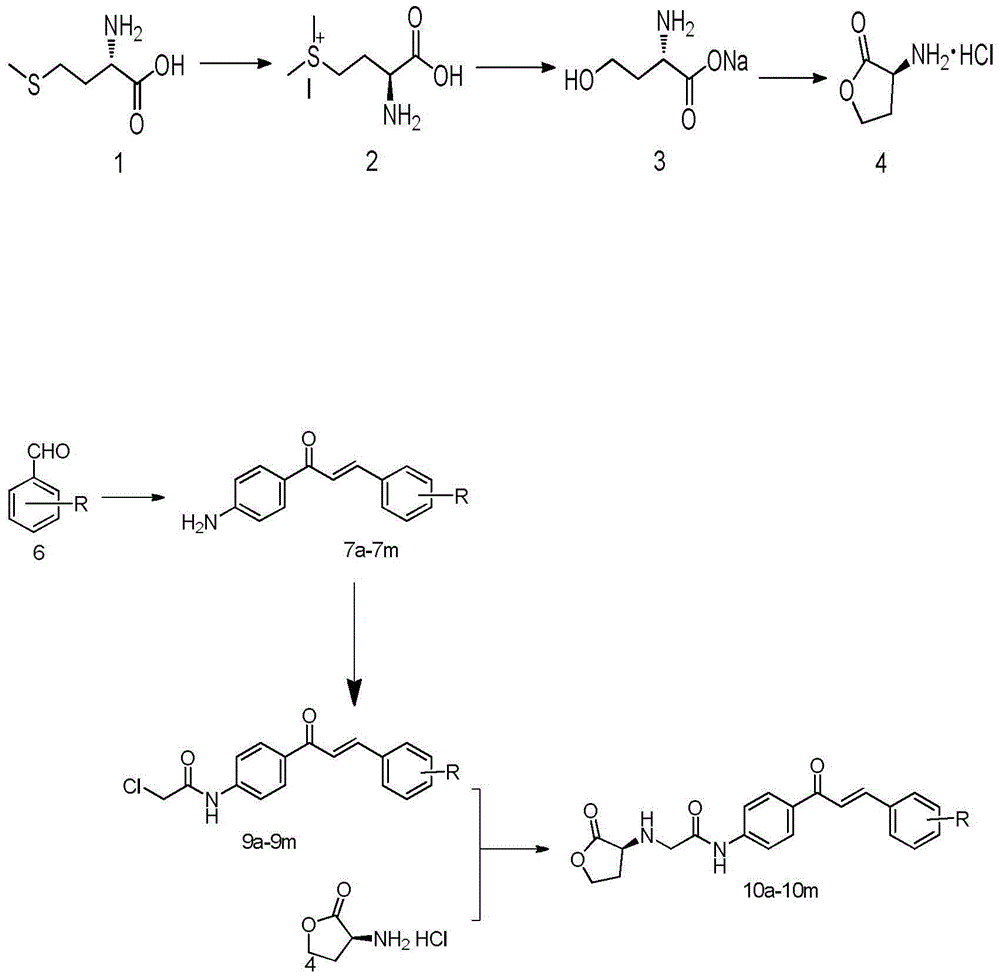
Homoserine lactone compounds as well as preparation methods and application thereof
A technology of ester compounds and homoserine, which is applied in organic chemistry, drug combination, and pharmaceutical formulation, can solve the problems of difficult access to chiral raw materials, limited in-depth research, and complicated synthetic routes, so as to improve quorum sensing inhibition activity and yield. The effect of high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (S,E)-2-((4-(3-(4-chlorophenyl)acryloyl)phenyl)amine)-N-(2-carbonyltetrahydrofuran-3-yl)acetamide
[0054] Add 10.12g (67.7mmol) of L-methionine to a mixed solution of 200mL of water and 30mL of methanol. After mixing well, slowly add about 5.1mL (1.2eq) of methyl iodide under airtight conditions. The reaction was stirred overnight until the system became clear and transparent. After concentrating under reduced pressure, add NaHCO dropwise to the system 3 Aqueous solution (5.69g is dissolved in 80mL water), after dropping, the system is transferred to an oil bath and heated to reflux for about 6 hours. After the TLC detection reaction is complete, the solvent is evaporated under reduced pressure to obtain a bright yellow oil, acetone and ethanol ( V:V=1:9) recrystallized to obtain a large amount of white solid. Dissolve the white solid in 100 mL of hydrochloric acid (6mol L -1 ) solution, heated to reflux for about 10h, the system was wine red, and TLC detected that ...
Embodiment 2
[0059] (S,E)-N-(4-(3-(4-chlorophenyl)acryloylphenyl)-2-((2-carbonyltetrahydrofuran-3-yl)amino)acetamide
[0060] Add 10.12g (67.7mmol) of L-methionine to a mixed solution of 200mL of water and 30mL of methanol. After mixing well, slowly add about 5.1mL (1.2eq) of methyl iodide under airtight conditions. The reaction was stirred overnight until the system became clear and transparent. After concentrating under reduced pressure, add NaHCO dropwise to the system 3 Aqueous solution (5.69g is dissolved in 80mL water), after dropping, the system is transferred to an oil bath and heated to reflux for about 6 hours. After the TLC detection reaction is complete, the solvent is evaporated under reduced pressure to obtain a bright yellow oil, acetone and ethanol ( V:V=1:9) recrystallized to obtain a large amount of white solid. Dissolve the white solid in 100 mL of hydrochloric acid (6mol L -1 ) solution, heated to reflux for about 10h, the system was wine red, and TLC detected that t...
Embodiment 3
[0118] The target compound synthesized by the present invention is tested for activity against two tumor cell lines of human liver cancer cell HePG2 and human esophageal cancer cell EC-109, and the test result shows that the derivative has certain antitumor activity.
[0119] 1. Experimental method:
[0120] (1) Preparation of 1640 culture medium: under sterile conditions, take an appropriate amount of serum-free RPMI 1640 medium, add it to 10% fetal bovine serum and shake evenly; then add double antibody (streptomycin 100 μg / mL and penicillin 100 μg / mL) and shake well. Store in the refrigerator at 4°C for later use.
[0121] (2) Preparation of PBS buffer salt: Weigh 1.56g Na2HPO4, 0.2g KH2PO4, 0.2g KCl, 8.0g NaCl, dissolve in 950mL ultrapure water, stir and dissolve with a clean glass rod, and then add ultrapure Dilute to 1000mL with water. Place it in a clean infusion bottle, insert a needle into the bottle stopper, sterilize at 121°C for 20 minutes under high temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com