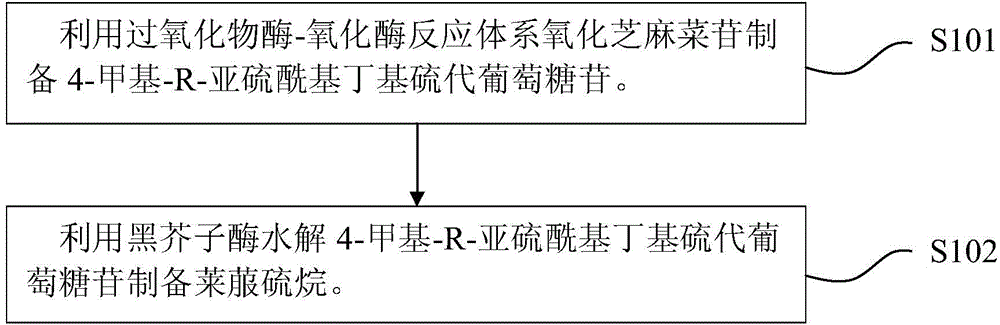
Method for preparing sulforaphane by using roquette seed glycosides
A technology of arugin and sulforaphane, which is applied in the field of biochemistry, can solve the problems of complex process, low yield, and many by-products, and achieve the effect of simple process, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of arugin: take 100 g of arugula seeds, inactivate enzymes at 100°C for 30 minutes, pulverize, add 1L of phosphate buffer, pH value of 7, and stir under boiling water for 1 hour, and filter with suction. The filtrate was applied to a D261 resin column with a column volume of 100ml, 500ml of deionized water to wash impurities, 500ml of 2M potassium chloride solution to wash the arugin product, 40°C rotary evaporation to remove the solvent, 500ml methanol extraction desalination, 40°C rotary evaporation to remove the solvent to obtain sesame seeds The crude cabbage glycoside is 9.87g.
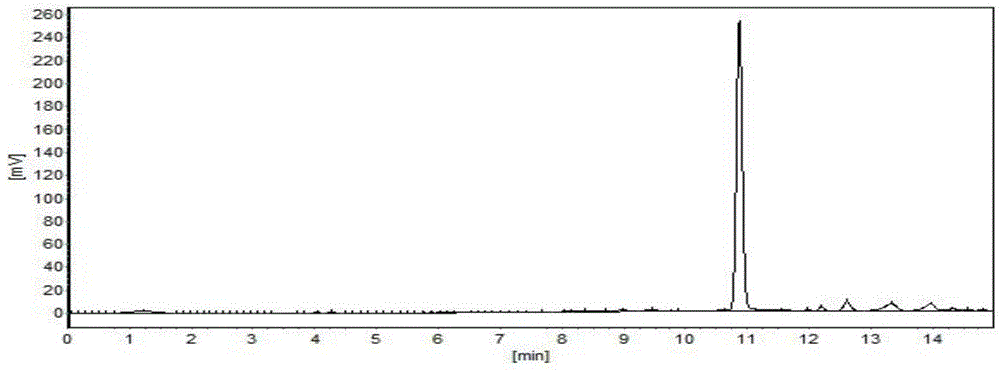
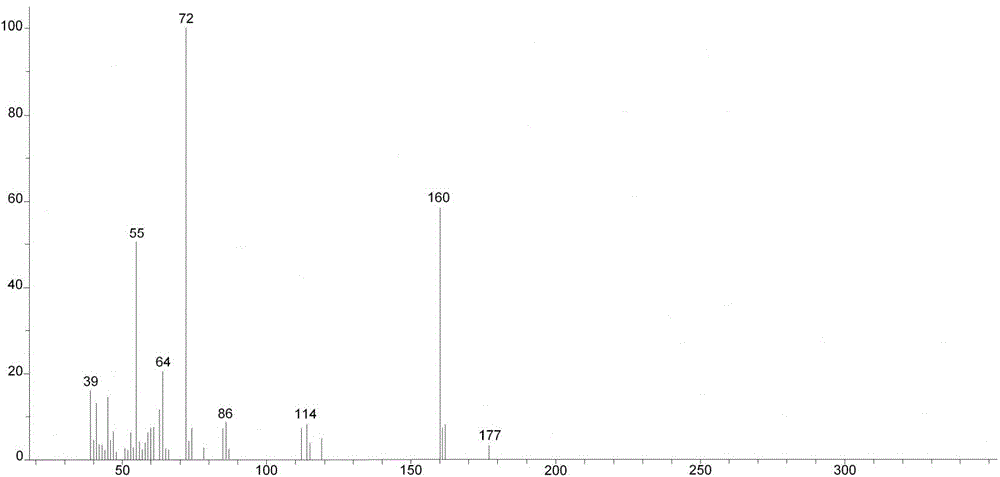
[0036] Preparation of 4-methyl-R-sulfinyl butyl glucosinolate: dissolve the crude arugin in 500ml phosphate buffer (pH 7), add glucose oxidase 0.0006mmol horseradish peroxide Enzyme 0.0024mmol, sealed with oxygen (1ml / min), controlled temperature at 28°C, and stirred with a magnetic stirrer. After 15 minutes, 1.4 mol glucose was added, and the reaction was stirred for 4 hours. T...
Embodiment 2
[0039] Preparation of arugin: take 100 g of arugula seeds, inactivate enzymes at 100°C for 30 minutes, pulverize, add 1L of phosphate buffer, pH value of 7, and stir under boiling water for 1 hour, and filter with suction. The filtrate was applied to a D261 resin column with a column volume of 100ml, 500ml of deionized water to wash impurities, 500ml of 2M potassium chloride solution to wash the arugin product, 40°C rotary evaporation to remove the solvent, 500ml methanol extraction desalination, 40°C rotary evaporation to remove the solvent to obtain sesame seeds Crude cabbage glycosides 10.12g.
[0040] Preparation of 4-methyl-R-sulfinyl butyl glucosinolate: dissolve the crude arugin in 500ml phosphate buffer (pH 7), add glucose oxidase 0.0006mmol horseradish peroxide Enzyme 0.0024mmol, sealed with oxygen (1ml / min), controlled temperature at 28°C, and stirred with a magnetic stirrer. After 15 minutes, 1.4 mol glucose was added, and the reaction was stirred for 2 hours. The fo...
Embodiment 3
[0043] Preparation of arugin: Take 1000g of arugula seeds, inactivate enzymes at 100°C for 30 minutes, pulverize, add 10L of phosphate buffer, pH value of 7, and stir under boiling water for 1 hour, and filter with suction. The filtrate was applied to a D261 resin column with a column volume of 1000ml, 5000ml of deionized water to wash impurities, 5000ml of 2M potassium chloride solution to wash the arugin product, 40℃ rotary evaporation to remove the solvent, 5000ml methanol extraction desalination, 40℃ rotary evaporation to remove the solvent to obtain sesame seeds The crude cabbage glycoside is 97.28g.
[0044] Preparation of 4-methyl-R-sulfinyl butyl glucosinolate: add arugula glycoside crude product to 500ml phosphate buffer (pH value 7), add glucose oxidase 0.006mmol chloroperoxidase 0.024 mmol, control the temperature at 31°C, use a magnetic stirrer. After 15 minutes, 14 mol glucose was added, and the reaction was stirred for 4 hours. The formation of reactants was detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com