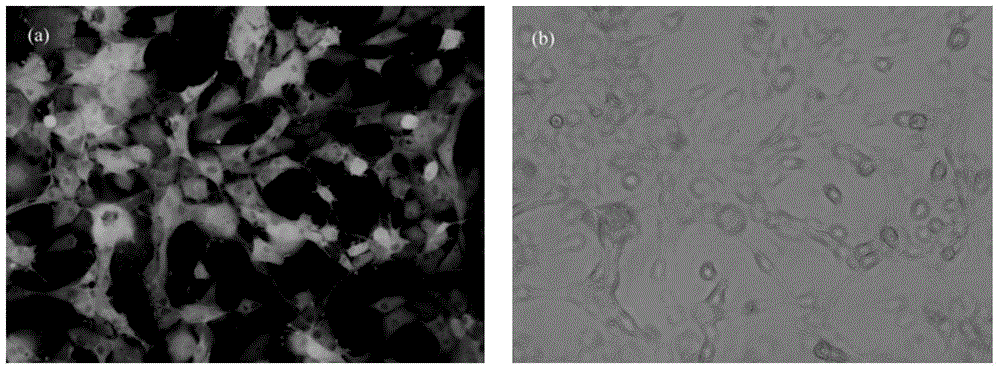
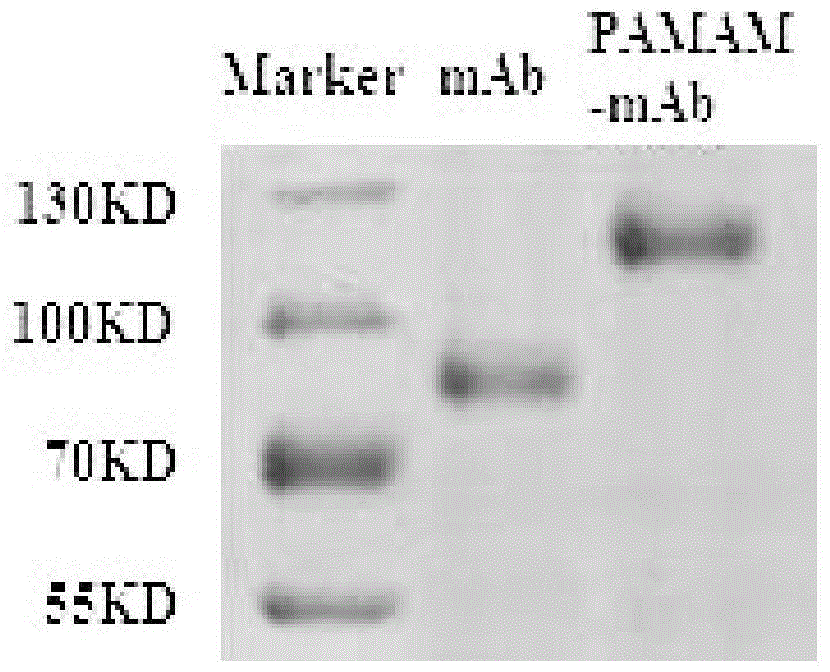
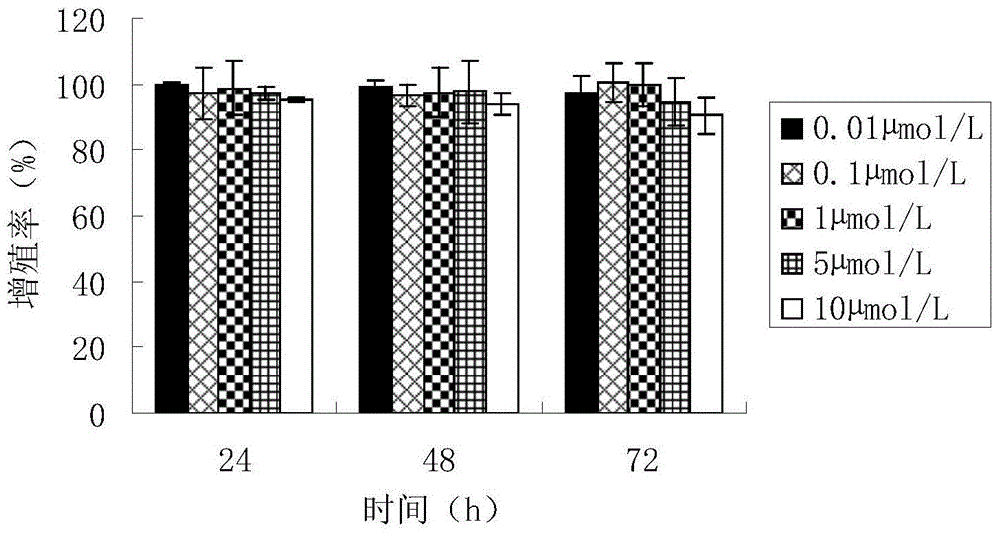
Targeted boron preparation and preparation method
A targeting and preparation technology, applied in the field of biomedicine, can solve the problems of low enrichment and poor targeting, and achieve the effects of low cytotoxicity, high encapsulation rate and reduced damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention also provides a preparation method of the targeting boron preparation.
[0046] A kind of preparation method of targeting boron preparation, by 3-(2-pyridine dimercapto) propionic acid N-hydroxysuccinimide ester and undecanoic acid maleimide-hydrazide-trifluoroacetic acid salt The amide-amine dendrimer and the epidermal growth factor antibody are connected to form a functionalized dendrimer, and then the polyhedral borane is loaded to obtain the targeted boron preparation.
[0047] N-hydroxysuccinimide 3-(2-pyridyldimercapto)propionate (SPDP), the molecular formula is C 12 h 12 N 2 o 4 S 2 , is a short-chain cross-linking agent, which connects the amino group on the PAMAM molecule through the hydroxysuccinimide ester active group (-NH 2 ). The molecular formula of undecanoic acid maleimide-hydrazide-trifluoroacetate (KMUH) is C 15 h 25 N 3 o 3 · CF 3 CO 2 H, is a long-chain cross-linking agent, which reacts with the sulfhydryl group (-SH) on th...
Embodiment 1
[0108] Example 1: Preparation of PAMAM-mAbEGFR / BSH
[0109] (1) Mix 0.1 mg of the epidermal growth factor receptor monoclonal antibody mAbEGFR with 2 mg of sodium periodate in 1 ml of 0.1 M acetate buffer at pH 4.5, use magnetic beads to keep stirring at room temperature for 2 h, and pass the reaction mixture through Sephadex Desalting the G25 chromatography column, removing excess sodium periodate, eluting with 50mM phosphate buffer at pH 7.5, and collecting the oxidized EGFR monoclonal antibody obtained;
[0110] (2) 0.58ml of 5% polyamidoamine dendrimer (PAMAM) solution (Sigma Company), which contains 29mg of PAMAM, is mixed with 3.12mg SPDP in 1ml of 0.1M phosphate buffered saline buffer solution at pH 7.5, and reacted at room temperature 2h, the reaction mixture was desalted by Sephadex G25 chromatography column to remove unreacted SPDP, and eluted with 50mM phosphate buffer solution with pH 7.5 to obtain the intermediate PAMAM-SPDP;
[0111](3) Dissolve 1.54mg of DTT in...
Embodiment 2
[0119] Embodiment 2: Preparation of PAMAM-mAbEGFR / BSH
[0120] (1) mAbEGFR 0.3mg and sodium periodate 6mg were mixed in 3ml of 0.1M acetate buffer solution with a pH of 4.5, stirred continuously at room temperature for 2 hours using magnetic beads, and the reaction mixture was desalted by Sephadex G25 chromatography column to remove Excess sodium periodate was eluted with 50 mM phosphate buffer at pH 7.5, and the resulting oxidized EGFR monoclonal antibody was collected;
[0121] (2) 1.74ml of polyamidoamine dendrimer PAMAM (Sigma company) solution, which contains 87mg of PAMAM, is mixed with 9.36mg of SPDP in 3ml of 0.1M phosphate buffer saline at pH7.5, reacted for 2h, reacted The mixture was desalted by Sephadex G25 chromatographic column to remove unreacted SPDP, and eluted with 50mM phosphate buffer at pH 7.5 to obtain the intermediate PAMAM-SPDP;
[0122] (3) 4.62 mg of DTT was dissolved in 3 ml of 0.1 M phosphate buffer solution with pH 7.5, and 0.3 g of DMSO was added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com