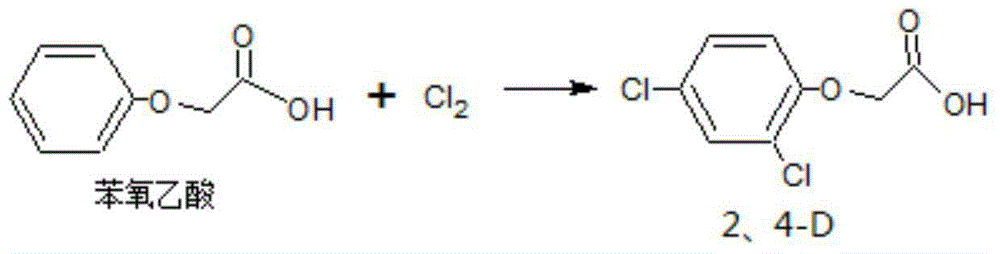2,4-dichlorphenoxyacetic acid preparation method
A technology of dichlorophenoxyacetic acid and phenoxyacetic acid, which is applied in the direction of carboxylate preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problems of complex synthetic route process, affecting quality and yield, etc., to achieve Effects of shorter reaction time, lower cost, and less loss of chlorine gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of phenoxyacetic acid
[0024] The mass ratio of chloroacetic acid, sodium hydroxide, and phenol is 1: 0.1: 0.8; the mass ratio of the acid and water is 3: 1; the mass ratio of the phenoxyacetic acid and chlorine is 1: 2.2
[0025] Add 1Kg of chloroacetic acid and 1Kg of 10% sodium hydroxide solution into the reactor, stir for 15-20 minutes, then add 800g of phenol 0.6mol, adjust the pH value to 11 with sodium bicarbonate; then heat at 100°C for 40 Minutes; then add hydrochloric acid to adjust the pH value to 4; finally cool in an ice-water bath, wash and dry the obtained solid to obtain phenoxyacetic acid with a content of 96%.
[0026] Preparation of Ni-Al-Mg Composite Oxide
[0027] Add 10g of nickel nitrate, 5g of magnesium nitrate, and 2g of aluminum nitrate to 50g of water respectively to obtain three aqueous solutions; mix the three aqueous solutions at 50°C; add 3g of sodium bicarbonate after half an hour; then stir at 50°C to obtain a precipitate; ...
Embodiment 2
[0028] Embodiment two: the preparation of 2,4-dichlorophenoxyacetic acid
[0029] In the reaction flask, put 10 mol of the original powder of phenoxyacetic acid in Example 1, 700 g of acetic acid and 175 g of water, and 76 g of nickel-aluminum-magnesium composite oxide; after stirring, start feeding 22 mol of chlorine gas for chlorination reaction, and stir to heat up to 75 ° C. Continue to stir for 20 minutes; then the reaction product is directly cooled to 18°C, kept for about 0.6 hours, filtered with suction, and the obtained filter cake is directly dried at 60°C to obtain 2,4-dichlorophenoxyacetic acid with a content of 98.6% and a yield (relative Phenoxyacetic acid calculation) 98.1%.
Embodiment 3
[0030] Embodiment three: the preparation of 2,4-dichlorophenoxyacetic acid
[0031] Using the mother liquor obtained in Example 2, add 50 g of 60% acetic acid aqueous solution and 9 mol of phenoxyacetic acid powder, and follow the process and operation described in Example 1 to obtain 2,4-dichlorophenoxyacetic acid with a content of 98.3% and a yield of 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com