A kind of method for preparing high-purity fidaxomicin
A fidaxomicin, high-purity technology, applied in the preparation of sugar derivatives, chemical instruments and methods, organic chemistry, etc., can solve the problems of large initial investment, high operating costs, and cumbersome operations, and achieve low operating costs and reduced The effect of simple production cost and operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
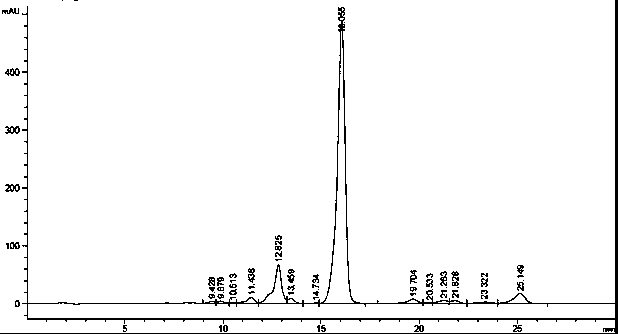
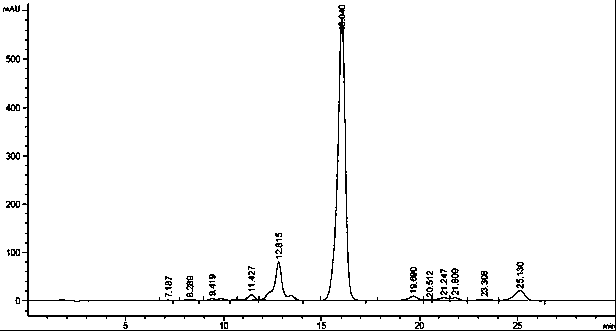
[0039] 80L fermented broth (HPLC chromatogram as figure 1Shown) pressure filtration obtains 28.5kg mycelia. Add 90L of 70% ethanol solution to the mycelium, stir for 5 hours, and filter to obtain 94.3L of filtrate, which contains 187g of fidaxomicin through HPLC detection. Dilute the filtrate with purified water to an ethanol concentration of 50%, then introduce it into SP825L adsorption resin with a capacity of 20L, pre-wash the resin with 40L of 60% ethanol solution at a flow rate (1BV / h), and then wash it with 100L of 70% ethanol solution Desorption, flow rate (1BV / h), collect one component per 2L, and mix the components with a purity above 70%. The mixed components were then concentrated under reduced pressure to an ethanol concentration of 13%. Stand still and cool to 15°C, filter with suction, and dry to obtain 224g yellow solid, yield 71.8%, HPLC: 76.7% (HPLC such as figure 2 shown).
Embodiment 2
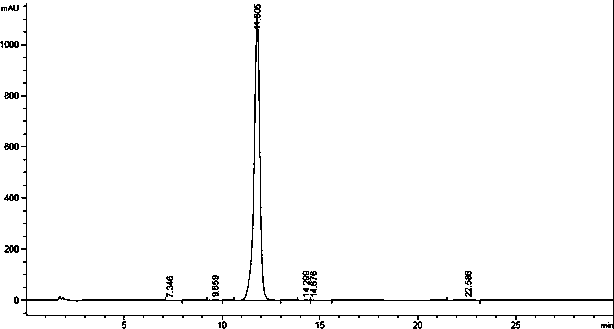
[0041] Dissolve 224g of the yellow solid obtained in Example 1 in 3.5L of ethyl acetate, dissolve, cool down to 5°C, drop 2.3L of 5°C petroleum ether into the above-mentioned ethyl acetate solution under stirring, and keep The temperature was stirred at 3-8°C for 5 hours. Filter, filter cake is vacuum-dried, obtain off-white powder 192g, yield 85%, HPLC: 77.3% (HPLC chromatogram such as image 3 shown).
Embodiment 3
[0043] 40 g of the off-white powder obtained in Example 2 was dissolved with 1 L of 50% acetonitrile aqueous solution into the upper column liquid, and introduced into UniPS40 resin for adsorption. The resin loading was 5 L, and the upper column flow rate was 5 L / h. Then use 40L of 65% acetonitrile aqueous solution to desorb, collect in sections, and mix the components with a purity of 95% or more. Concentrate the mixture in vacuo at 50-60°C until a solid precipitates out, then stop the concentration, cool to room temperature, add ethyl acetate to stir, dissolve, extract, and discard the water layer. The combined ethyl acetate layers were concentrated to dryness to obtain a white solid, which was dissolved by adding ethanol and heated to 50°C. The amount of ethanol was 12 times the weight of the white solid, and the temperature was lowered to 5°C while stirring. A white solid was precipitated, and the stirring was continued until the precipitated solid disappeared. Add more, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com