Edible composition and use thereof
A composition and food technology, applied in the directions of food science, bacteria used in food preparation, applications, etc., can solve problems such as undeveloped combined drug delivery, and achieve the improvement of human immunity, good safety, and improved immunity of the body. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 probiotic culture medium
[0021] 1. Culture medium of Clostridium butyricum and Clostridium butyricum:
[0022] Tryptic casein: 5.0g
[0023] Peptone (pepsin digested): 5.0g
[0024] Yeast paste: 10.0g
[0025] Resazurin: 1.0mg
[0026] CaCl 2 x2H 2 O: 0.25g
[0027] MgSO 4 wxya 2 O: 0.5g
[0028] K 2 HPO 4 : 1.0g
[0029] K H 2 PO 4 : 1.0g
[0030] NaHCO 3 : 10.0g
[0031] NaCl: 2.0g
[0032] Cysteine-HCl x H 2 O: 0.5g
[0033] ddH 2 0 to 1L, adjust the pH to 7.0.
[0034] 2. The third Clostridium medium:
[0035] Beef extract: 20.0g
[0036] Peptone: 30.0g
[0037] Yeast paste: 5.0g
[0038] K 2 HPO 4 : 5.0g
[0039] Resazurin: 1.0mg
[0040] Glucose: 4.0g
[0041] Cellobiose: 1.0g
[0042] Maltose: 1.0g
[0043] Soluble starch: 1.0g
[0044] ddH 2 O was adjusted to 1L, and the pH was adjusted to 7.0.
[0045] 3. Rose Berry culture medium
[0046] Tryptic casein: 10.0g
[0047] Yeast paste: 5.0g
[0...
Embodiment 2
[0130] The preparation of embodiment 2 probiotics and composition thereof
[0131] 1. Preparation of Live Clostridium Butyricum Capsules
[0132] Cultivate Clostridium butyricum species with liquid or solid medium, collect and wash the bacteria, dry to prepare active bacteria powder, use gelatin to make capsule material, use PVP as the subcoat, and then use beeswax for outer coating. No. 0 capsule packaging, get Clostridium butyricum capsules, the number of viable bacteria is not less than 1.0*10 8 CFU / g.
[0133] 2. Preparation of live Clostridium butyricum capsules containing polyfructose
[0134] Cultivate Clostridium butyricum with liquid or solid medium, collect and wash the bacteria, dry to prepare active bacteria powder, add polyfructose according to the number of viable bacteria of Clostridium butyricum, so that the number of viable bacteria is not less than 1.0* 10 8CFU / g, the amount of polyfructose added is not less than 4g, the capsule material is made of gelati...
Embodiment 4
[0202] Example 4 Probiotic composition lowering blood sugar, lowering blood fat experiment
[0203] According to the Ministry of Health's "Health Food Inspection and Evaluation Technical Specifications (2003)", the evaluation method of the auxiliary blood sugar and blood fat lowering function human body test food experiment evaluation method is carried out.
[0204] 1. Preparation of probiotic composition:
[0205] According to Example 1, three different compositions of probiotic single powder (capsule), bacterial powder and polyfructose (capsule), bacterial powder and culture solution (oral solution) were prepared.
[0206] 2. Human food trial and test method:
[0207] A total of 270 patients with type Ⅱ diabetes mellitus were selected on a voluntary basis, including 200 males and 70 females, aged 43-75 years, without serious complications such as heart, liver and kidney.
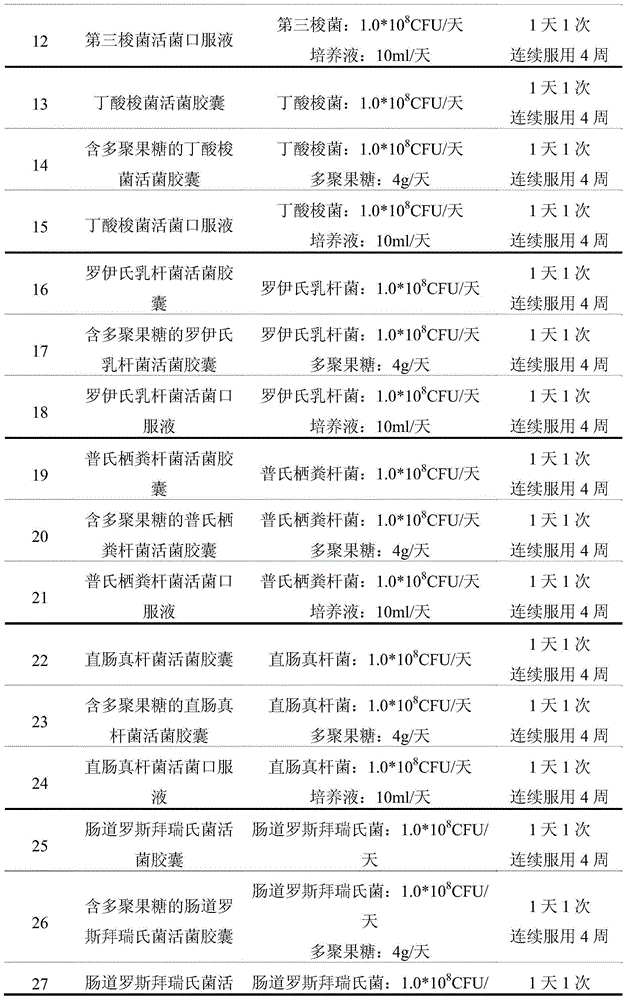
[0208] The subjects were randomly divided into 27 groups, with 10 people in each group. The types of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com