Oral solid preparation released at given time and preparation method thereof
A preparation and filler technology, which is applied in the field of time-selected release oral solid preparations and preparations, can solve the problems of patient inconvenience, reduction of drug therapeutic effect, and impact on effectiveness, so as to increase sleep time, improve therapeutic effect, and reduce adverse reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
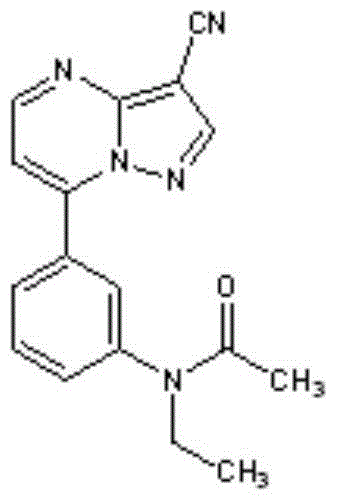
[0051] Example 1: Zaleplon timed release formulation and preparation method thereof
[0052] The preparation method of zaleplon time-release preparation, the preparation method firstly adopts a tablet press or a dry granulator to extrude to obtain drug-containing tablet cores or granules according to the formula, and the drug-containing tablet cores or granules are prepared by coating pan or fluidized Bed coating, the coating film is attached to the tablet core or granule containing the drug, that is, the timed release preparation of zaleplon is obtained;
[0053] The composition of raw materials and auxiliary materials of the timed-release preparation of zaleplon is:
[0054] Zaleplon 60g, filler lactose 780g, binder povidone 62.5g, disintegrant crospovidone 108g, lubricant sodium stearate fumarate 12g, hypromellose 240g, behenic acid Glycerides 600g, sunscreens are titanium dioxide 600g and 95% ethanol 10000ml.
[0055] Various raw and auxiliary materials are prepared acco...
Embodiment 2
[0058] Example 2: Zaleplon timed release formulation and preparation method thereof
[0059] The preparation method of zaleplon time-release preparation, the preparation method firstly adopts a tablet press or a dry granulator to extrude to obtain drug-containing tablet cores or granules according to the formula, and the drug-containing tablet cores or granules are prepared by coating pan or fluidized Bed coating, the coating film is attached to the tablet core or granule containing the drug, that is, the timed release preparation of zaleplon is obtained;
[0060] Basically the same as Example 1, the difference is:
[0061] The composition of raw materials and auxiliary materials of the timed-release preparation of zaleplon is:
[0062] Zaleplon 90g, Filler Calcium Hydrogen Phosphate 840g, Disintegrant Crospovidone 72g, Lubricant Magnesium Stearate 6g, Lubricant Micropowder Silica Gel 6g, Hypromellose 180g, Glyceryl Behenate 720g, The sunscreen is 360g of titanium dioxide an...
Embodiment 3
[0064] Example 3: Zaleplon timed release formulation and preparation method thereof
[0065] The preparation method of zaleplon time-release preparation, the preparation method firstly adopts a tablet press or a dry granulator to extrude to obtain drug-containing tablet cores or granules according to the formula, and the drug-containing tablet cores or granules are prepared by coating pan or fluidized Bed coating, the coating film is attached to the tablet core or granule containing the drug, that is, the timed release preparation of zaleplon is obtained;
[0066] Basically the same as Example 1, the difference is:
[0067] The composition of raw materials and auxiliary materials of the timed-release preparation of zaleplon is:
[0068] Zaleplon 125g, Filler Mannitol 500g, Disintegrant Croscarmellose Sodium 115g, Binder Hypromellose 62.5g, Lubricant Magnesium Stearate 12.5g, Hypromellose 225g , 550g of glyceryl behenate, 250g of titanium dioxide and 5000ml of 85% ethanol as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com