Nifuratel nysfungin vaginal soft capsule and preparation process thereof
A technology of nifuratel nystatin and soft capsules is applied in the field of pharmaceutical preparations, which can solve the problems of lack of credibility, instability of nystatin to water, imbalance of beneficial vaginal flora and the like, and achieves improved preparation process, Improve drug stability and drug efficacy, promote the effect of restoring balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
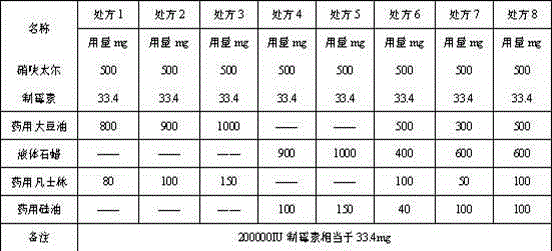
[0057] Calculated according to the weight of 1000 capsules, the content components are: 500g of nifuratel, 33.4g of nystatin, 900g of soybean oil, and 100g of medicinal vaseline. The capsule shell components of the soft capsule are: 380g of gelatin, 182.4g of glycerin, 380g of water, 1g of sunset yellow, 2g of titanium dioxide, and 2g of ethylparaben.
[0058] According to the above formula, the operation process: (1) pre-treatment of raw materials, after passing nifuratel and nystatin through a 120-mesh sieve, and strictly avoiding light, take the prescription amount for later use; (2) prepare the prescription amount Vaseline, nifuratel, and nystatin are sequentially added into a stirring and insulating barrel filled with medicinal soybean oil, the temperature is 40°C, and the stirring speed is 800-1500rpm, until they are uniform and ready for use; (3) Preparation of soft capsule shell materials, ① Add the prescribed amount of ethylparaben, sunset yellow, and titanium dioxide...
Embodiment 2
[0060] Calculated according to the weight of 1000 capsules, the content components are: 500g of nifuratel, 33.4g of nystatin, 1000g of soybean oil, and 150g of medicinal vaseline. The capsule shell components of the soft capsule are: 380g of gelatin, 182.4g of glycerin, 380g of water, 1g of sunset yellow, 2g of titanium dioxide, and 2g of ethylparaben.
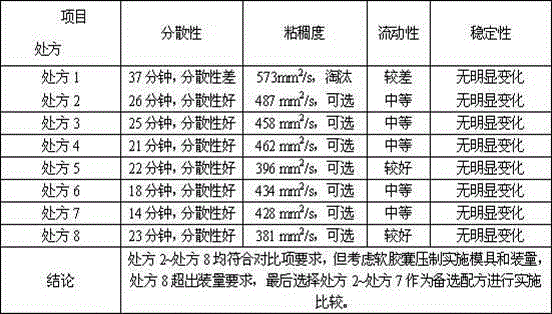
[0061] According to the above formula, adopt the same preparation method as Example 1 to prepare soft capsules. The inspection results are shown in Table 6.
Embodiment 3
[0063] Calculated according to the weight of 1000 capsules, the content components are: 500g of nifuratel, 33.4g of nystatin, 1000g of liquid paraffin, and 150g of medicinal vaseline. The capsule shell components of the soft capsule are: 380g of gelatin, 182.4g of glycerin, 380g of water, 1g of tartrazine, 2g of titanium dioxide, and 2g of methylparaben.
[0064] According to the above formula, adopt the same preparation method as Example 1 to prepare soft capsules. The inspection results are shown in Table 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com