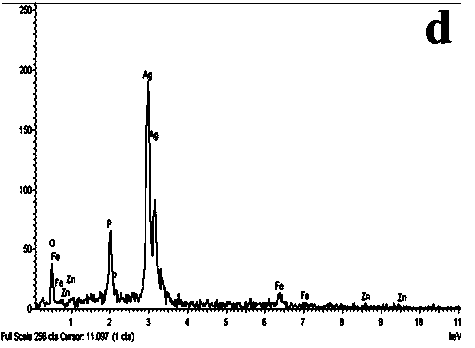
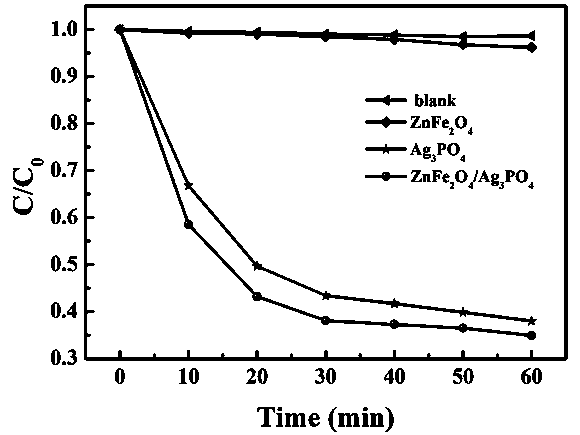
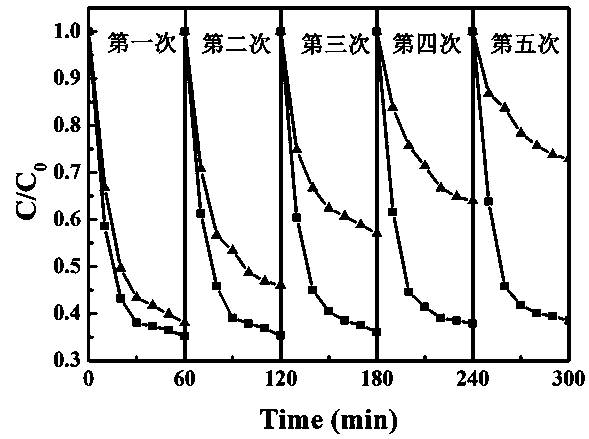
Preparation method of ZnFe2O4/ Ag3PO4 composite photocatalyst
A technology of znfe2o4 and composite light, which is applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of silver phosphate visible light catalytic activity and stability improvement, limited and incomplete ability to conduct photogenerated electrons, etc. problem, to promote electron transfer and free radical generation, facilitate rapid recovery and efficient utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Weigh FeCl 3 ·6H 2 O solid 0.54g and ZnCl 2 Add 0.136g of solid to 50mL of ethylene glycol and stir evenly, then add 3.6g of NaAC and 1g of PEG and continue stirring until uniform. Transfer the mixed solution to a reaction kettle and react at 200°C for 10 hours to obtain ZnFe 2 o 4 nanospheres. Separation by magnetic separation, washing with deionized water and ethanol three times each, and vacuum drying at 50°C.
[0031] (2) Weigh 8.493g of silver nitrate solid and dissolve it in 250mL deionized water to make a silver nitrate solution with a concentration of 0.2M; 2 HPO 4 12H 2 17.907g of O solid (equivalent to 7.093g of disodium hydrogen phosphate) was dissolved in 250mL of deionized water to prepare a 0.2M disodium hydrogen phosphate solution.
[0032] (3) Weigh the ZnFe prepared in step (1) 2 o 4 Add 0.0482 g of nanospheres into 60 mL of deionized water, and sonicate for 60 min. Stir rapidly with a mechanical stirrer and add 3 mL of the disodium hydro...
Embodiment 2
[0037] (1) Weigh FeCl 3 ·6H 2 O solid 0.135g and ZnCl 2 Add 0.043g of solid to 50mL of ethylene glycol and stir evenly, then add 0.9g of NaAC and 0.25g of PEG and continue stirring until uniform. Transfer the mixed solution to the reaction kettle and react at 180°C for 8 hours to obtain ZnFe 2 o 4 nanospheres. Separation by magnetic separation, washing with deionized water and ethanol three times each, and vacuum drying at 50°C.
[0038] (2) Weigh 2.123g of silver nitrate solid and dissolve it in 250mL deionized water to make a silver nitrate solution with a concentration of 0.05M; 2 HPO 4 12H 2 O solid 4.476g (equivalent to disodium hydrogen phosphate 1.773g) was dissolved in 250mL deionized water to prepare a disodium hydrogen phosphate solution with a concentration of 0.05M.
[0039] (3) Weigh the ZnFe prepared in step (1) 2 o 4 Add 0.0241 g of nanospheres into 60 mL of deionized water, and sonicate for 60 min. Stir rapidly with a mechanical stirrer and add 1 mL ...
Embodiment 3
[0041] (1) Weigh FeCl 3 ·6H 2 O solid 1.08g and ZnCl 2 Add 0.272g of solid to 50mL of ethylene glycol and stir evenly, then add 7.2g of NaAC and 2g of PEG and continue stirring until uniform. Transfer the mixed solution to a reaction kettle and react at 200°C for 12 hours to obtain ZnFe 2 o 4 nanospheres. Separation by magnetic separation, washing with deionized water and ethanol three times each, and vacuum drying at 50°C.
[0042] (2) Weigh 12.74g of silver nitrate solid and dissolve it in 250mL deionized water to make a silver nitrate solution with a concentration of 0.3M; 2 HPO 4 12H 2 26.11 g of O solid (equivalent to 10.639 g of disodium hydrogen phosphate) was dissolved in 250 mL of deionized water to prepare a disodium hydrogen phosphate solution with a concentration of 0.3M.
[0043] (3) Weigh the ZnFe prepared in step (1) 2 o 4 Add 0.1928g of nanospheres into 60mL of deionized water, and sonicate for 60min. Stir rapidly with a mechanical stirrer and add 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com