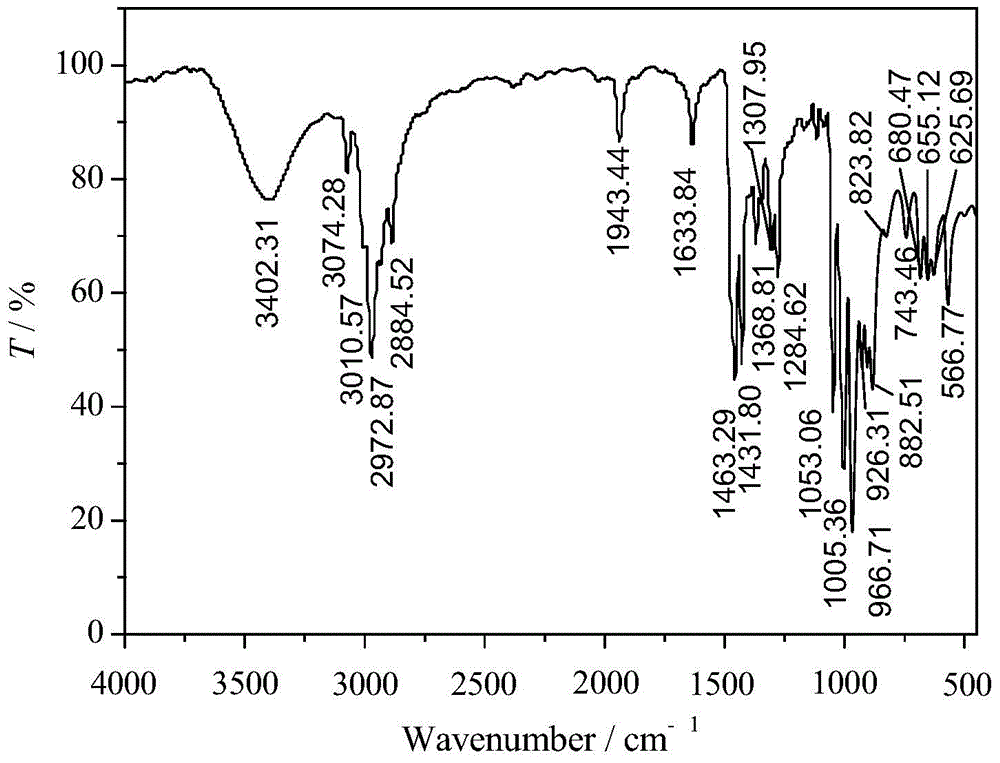
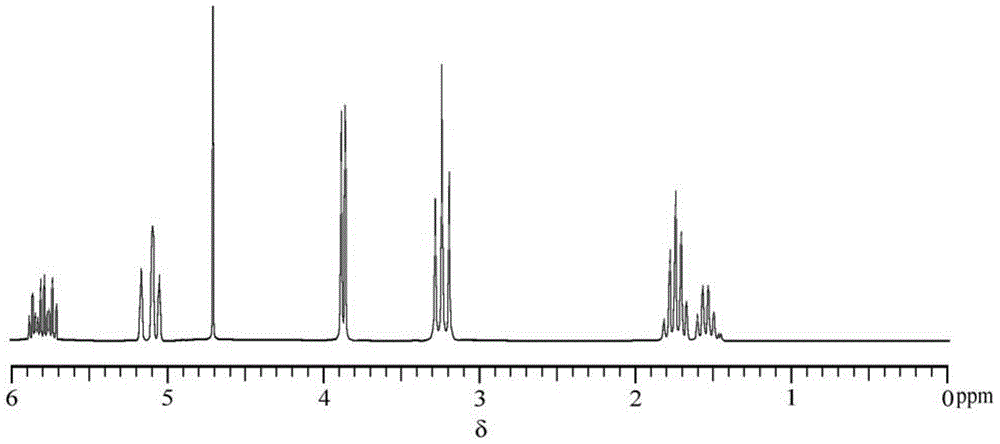
Preparation method of bromized N,N-diallyl piperidine onium salt cationic monomer
A technology of diallyl piperidine and cationic monomers, which is applied in the field of preparation of N,N-diallyl piperidinium bromide salts of cationic monomers, and can solve complicated operations, many side reactions, many steps, etc. problem, to achieve the effect of increasing the reaction speed, improving the utilization rate and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
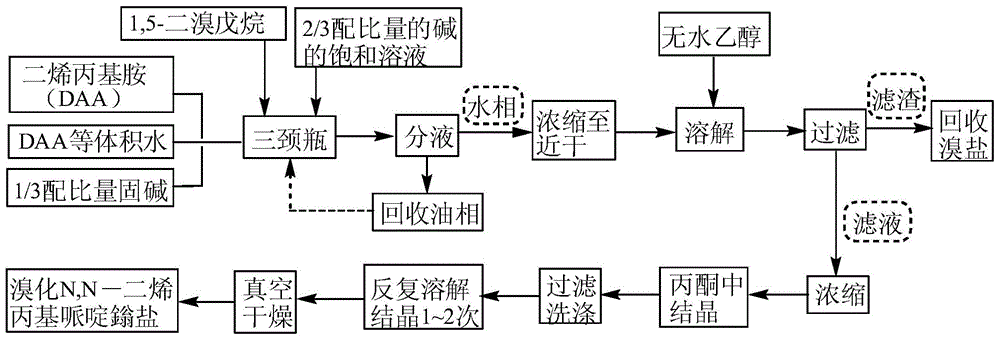
[0036] (1) The molar ratio of diallylamine, 1,5-dibromopentane and alkali is 4:1:1, and 54.2mL of diallyl and 5gK 2 CO 3 Add the solid to a 250mL three-neck flask equipped with a condenser, a constant pressure dropping funnel, and a mechanical stirrer, then add 40.7mL of water, stir evenly, raise the temperature to 60°C and start adding 15mL of 1,5-dibromopentane dropwise for 3 hours. Completed, continue to react for 3h, start to drop 19.2g K 2 CO 3 Saturated solution, after 2h addition, continue to react for 4h;
[0037] (2) The above-mentioned liquid phase was transferred into a separatory funnel, and after standing for 30 minutes, the liquid was separated to obtain 26 mL of the oil phase, which was recovered and entered into the next round of reaction;
[0038] (3) The water phase is evaporated and concentrated to nearly dryness, dissolved by adding absolute ethanol, and filtered to remove insoluble inorganic matter; evaporate the filtrate to a paste that can still flow,...
Embodiment 2
[0045] (1) Mix diallylamine, 1,5-dibromopentane and alkali in a molar ratio of 5:1:1.02, mix 67.8mL diallyl and 5.1g K 2 CO 3 Add the solid to a 250mL three-neck flask equipped with a condenser, a constant pressure dropping funnel and a mechanical stirrer, then add 67.8mL of water, stir evenly, raise the temperature to 65°C and start adding 15mL of 1,5-dibromopentane dropwise for 3.5h After dripping, continue to react for 2.5h, start to drop 19.5g K 2 CO 3 Saturated solution, after 2h addition, continue to react for 4h;
[0046] (2) The above-mentioned liquid phase was transferred into a separatory funnel, and after standing for 30 minutes, the liquid was separated to obtain 38.2 mL of the oil phase, which was recovered and entered into the next round of reaction;
[0047] (3) The water phase is evaporated and concentrated to nearly dryness, dissolved by adding absolute ethanol, and filtered to remove insoluble inorganic matter; evaporate the filtrate to a paste that can st...
Embodiment 3
[0051] (1) Mix diallylamine, 1,5-dibromopentane and alkali in a molar ratio of 6:1:1.05, mix 81.3mL diallyl and 5.24g K 2 CO 3 Add the solid to a 250mL three-necked flask equipped with a condenser, a constant pressure dropping funnel, and a mechanical stirrer, then add 81.3mL of water, stir evenly, raise the temperature to 70°C and start adding 15mL of 1,5-dibromopentane dropwise for 4 hours. Completed, continue to react for 2h, start to drop 20.1g K 2 CO 3 Saturated solution, after 2h addition, continue to react for 4h;
[0052] (2) The above-mentioned liquid phase was transferred into a separatory funnel, and after standing for 30 minutes, the liquid was separated to obtain 51.7 mL of the oil phase, which was recovered and entered into the next round of reaction;
[0053] (3) The water phase is evaporated and concentrated to nearly dryness, dissolved by adding absolute ethanol, and filtered to remove insoluble inorganic matter; evaporate the filtrate to a paste that can s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com