A kind of preparation method of dabigatran etexilate intermediate
A technology for dabigatran etexilate and intermediates, which is applied in the field of preparation of dabigatran etexilate intermediates, can solve the problems of product quality and yield, the reaction is easily affected by moisture, and the yield is not high, and the operation is achieved. The effect of reducing steps, strong selectivity, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
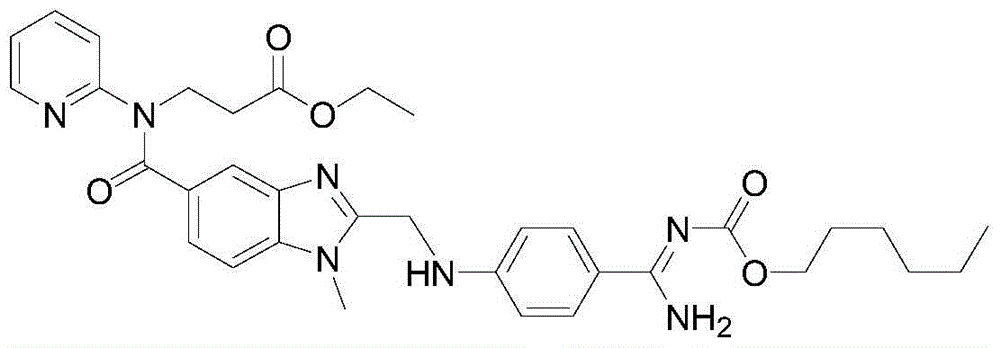
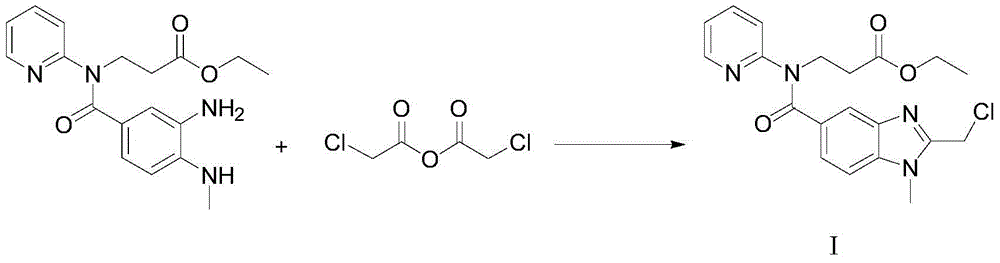
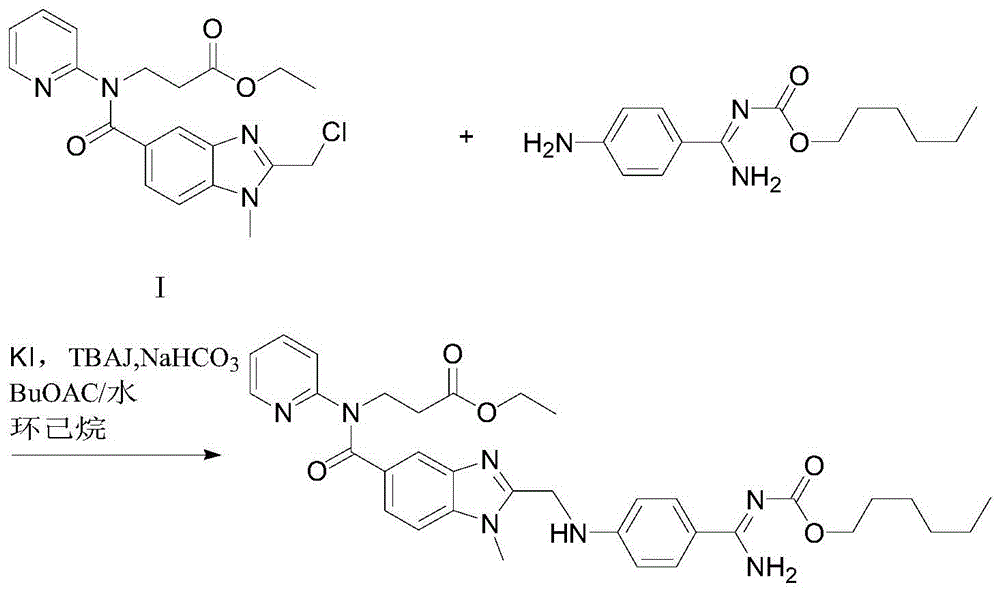
[0039] In this example, the intermediate of dabigatran etexilate β-alanine-N-[[1-methyl-1H-benzimidazole-2-chloromethyl]-5-carbonyl]-N-2-pyridine- The preparation method of ethyl ester includes the following steps:
[0040] (1) Intermediate 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamide]-ethyl acrylate 100g and ethyl chloroacetate 35.8g (molar ratio is 1 1) Add to 300ml of acetone and stir to dissolve, add 30g of the immobilized enzyme Novozym435 to the reaction solution, stir at 25°C for 15h, liquid phase detection of raw material reaction is complete;
[0041] (2) Remove the immobilized enzyme Novozym435 by filtration, evaporate the reaction solvent acetone, add 400ml ethyl acetate and 200ml purified water to wash and extract, and collect the organic layer;
[0042] (3) Evaporate the organic layer with ethyl acetate, and add the resulting oil to 200 ml of glacial acetic acid to react at 105°C for 2 hours;
[0043] (4) After the reaction is completed, remove the glacial acetic aci...
Embodiment 2
[0045] The difference between this embodiment and the embodiment 1 is only that the acetone in step (1) is replaced with tetrahydrofuran, the yield of the product obtained is 76%, and the liquid phase detection purity is 94%.
Embodiment 3
[0047] The difference between this embodiment and embodiment 1 is only that the acetone in step (1) is replaced with dichloromethane, the yield of the obtained product is 79%, and the purity of the liquid phase is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com