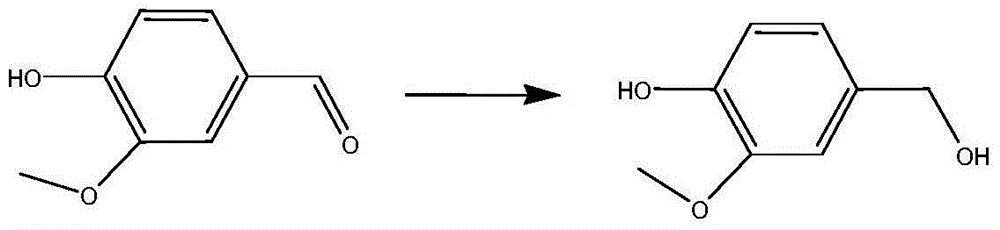
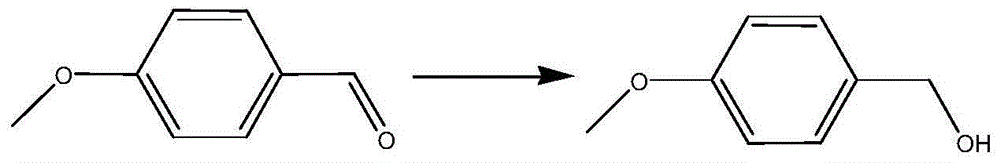
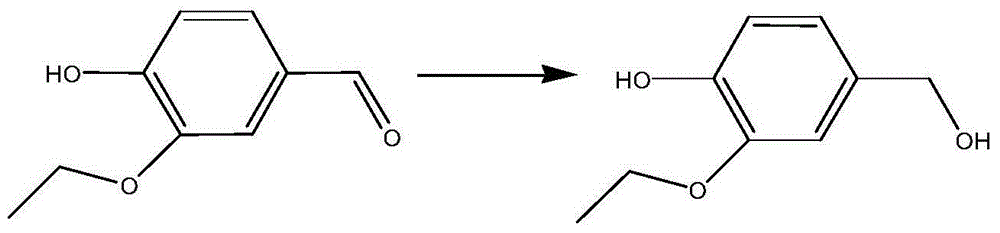
Ru-Co nanocatalyst and application of Ru-Co nanocatalyst in selective hydrogenation reaction of benzaldehyde compound
A compound, cobalt nanotechnology, applied in the ruthenium-cobalt nanocatalyst and its application in the selective hydrogenation reaction of benzaldehyde compounds, can solve the problems of troublesome preparation process of carbon nanotubes, difficulty in large-scale preparation and production, and achieve convenient reduction , easy to industrial production, good selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Take by weighing 0.027g ruthenium (III) chloride hydrate (ruthenium content 37%) in the round bottom flask, 20mL water is used as solvent, then add 1.05g cobalt nitrate, after all dissolving, slowly add 0.7g gac (noble metal ruthenium compound (calculated as Ru), the metal cobalt compound (calculated as Co) and the mass ratio of activated carbon is 1:21.3:70.1). After stirring for 3 hours, sodium hydroxide solution was added dropwise to make the pH = 11. After stirring for 12 hours, the solvent was filtered and washed with distilled water until pH = 7. The obtained supported pre-catalyst was vacuum-dried at 60° C. for 12 hours. The dried pre-catalyst was placed in a tube furnace and reduced under hydrogen at 300°C for 4h (heating rate 5°C / min). After naturally cooling down to room temperature, catalyst C1 was obtained.
Embodiment 2
[0021] Weigh 0.004g ruthenium (III) chloride hydrate (37% ruthenium content) in the round bottom flask, 20mL water is used as solvent, then add 0.525g cobalt nitrate, after all dissolving, slowly add 0.7g gac (noble metal ruthenium compound (calculated as Ru), the mass ratio of metal cobalt compound (calculated as Co) and activated carbon is 1:72.0:473.0). After stirring for 4 hours, potassium hydroxide solution was added dropwise to make the pH = 10. After stirring for 6 hours, the solvent was filtered and washed with distilled water until pH = 7.5. The obtained supported pre-catalyst was vacuum-dried at 40° C. for 12 hours. The dried pre-catalyst was placed in a tube furnace and reduced under hydrogen at 200°C for 4h (heating rate 8°C / min). After naturally cooling down to room temperature, catalyst C2 was obtained.
Embodiment 3
[0023] Take by weighing 0.027g ruthenium (III) chloride hydrate (ruthenium content 37%) in the round bottom flask, 20mL water is used as solvent, then add 1.75g cobalt nitrate, after all dissolving, slowly add 3.5g gac (noble metal ruthenium compound (calculated as Ru), the mass ratio of metal cobalt compound (calculated as Co) and activated carbon is 1:35.4:350.4). After stirring for 4 hours, sodium hydroxide solution was added dropwise to make the pH = 9. After stirring for 8 hours, the solvent was filtered and washed with distilled water until pH = 7. The obtained supported pre-catalyst was vacuum-dried at 80° C. for 12 hours. The dried pre-catalyst was placed in a tube furnace and reduced under hydrogen at 300°C for 4h (heating rate 5°C / min). After naturally cooling down to room temperature, catalyst C3 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com