Preparation method of terephthaloyl chloride
A technology for terephthaloyl chloride and terephthalic acid, which is applied in the field of preparation of terephthaloyl chloride, can solve the problems of instability of N,N-dimethylformamide, difficult separation of purity, long time and the like, and achieves The effect of easy exhaust, maintaining high purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
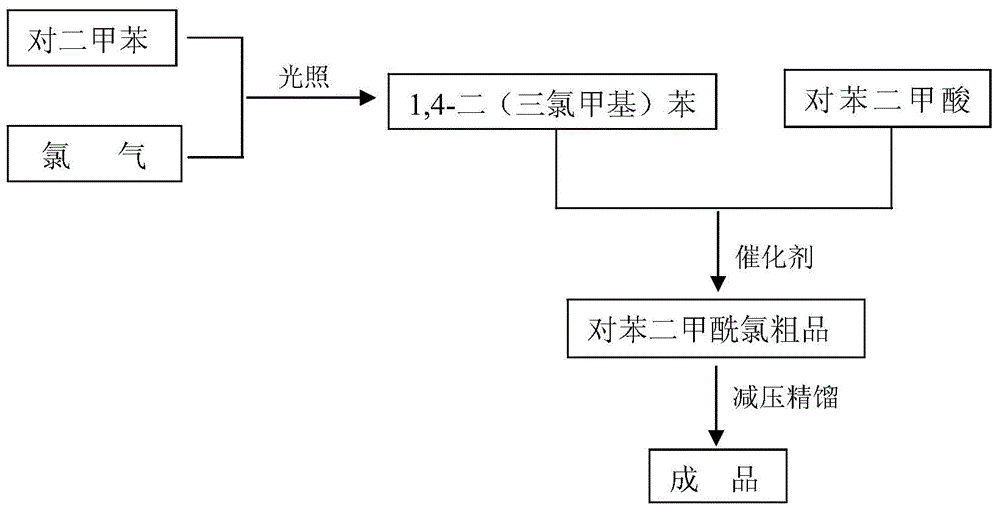
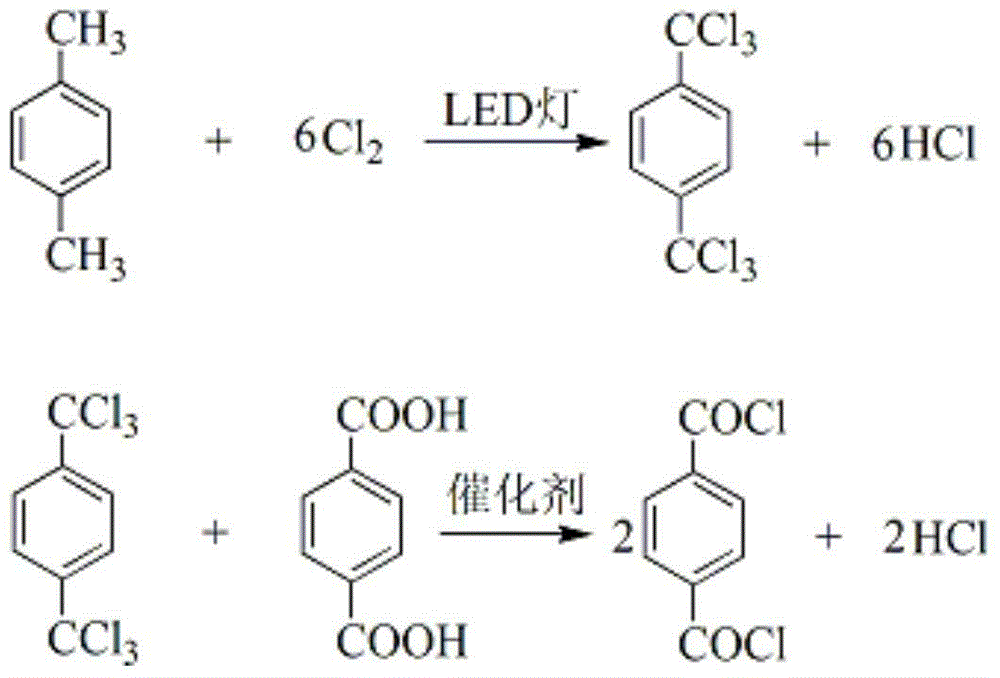
[0025] (1) Take 100g of p-xylene, under the irradiation of LED light source with a wavelength of 380-390nm, pass 481.5g of chlorine gas into it at 130°C, and react for 8 hours to obtain 1,4-bis(trichloromethyl)benzene Crude product 289.6g, chromatographic content is 92.0%;
[0026] (2) The 1,4-bis(trichloromethyl)benzene crude product obtained in step (1) and 144.8 g of terephthalic acid were added to the reaction flask, and under the catalysis of 0.145 g of zinc chloride, at 120° C. The reaction was carried out for 8 hours, until no hydrogen chloride gas was released, and the obtained reaction solution was rectified under reduced pressure to obtain 363.71 g of terephthaloyl chloride, the chromatographic content was 99.93%, the melting point was 82.3 ° C, and the total product yield was 95.1% (calculated by p-xylene). ).
Embodiment 2
[0028] (1) Take 100g of p-xylene, under the irradiation of LED light source with a wavelength of 420-430nm, pass 521.6g of chlorine gas into it at 140°C, and react for 9 hours to obtain 1,4-bis(trichloromethyl)benzene Crude product 290.27g, chromatographic content is 95.17%;
[0029] (2) 1,4-bis(trichloromethyl)benzene crude product obtained in step (1) and 153.8g of terephthalic acid were added to the reaction flask, and under the catalysis of 0.461g of ferric chloride, at 130°C The reaction was carried out for 7 hours until no hydrogen chloride gas was released, and the obtained reaction solution was rectified under reduced pressure to obtain 371.40 g of terephthaloyl chloride, the chromatographic content was 99.91%, the melting point was 82.2°C, and the product yield was 97.1% (calculated by p-xylene) .
Embodiment 3
[0031] (1) Take 100g of p-xylene, under the irradiation of LED light source with a wavelength of 470-480nm, pass 561.7g of chlorine gas into it at 150°C, and react for 10 hours to obtain 1,4-bis(trichloromethyl)benzene Crude product 290.34g, chromatographic content is 96.0%;
[0032] (2) The 1,4-bis(trichloromethyl)benzene crude product obtained in step (1) and 162.6 g of terephthalic acid were added to the reaction flask, and under the catalysis of 0.813 g of zinc chloride, at 140° C. React for 6 hours, until no hydrogen chloride gas is released, the gained reaction solution is rectified under reduced pressure to obtain terephthaloyl chloride 375.57g, the chromatographic content is 99.94%, the melting point is 82.5 ° C, and the total product yield is 98.2% (calculated by p-xylene). ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com