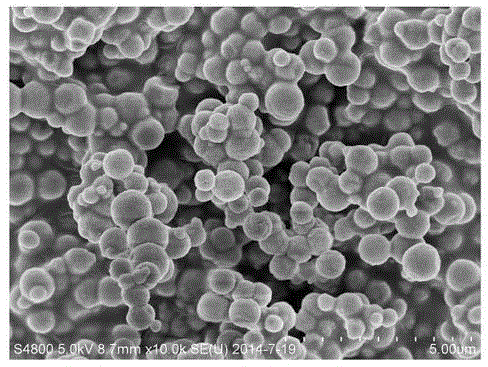
Preparation method of high-purity superfine cobalt oxalate powder
A fine grass, high-purity technology, applied in the field of metallurgy and chemical industry, can solve the problems of excessive particle growth, cumbersome process, increased particle and particle agglomeration, etc., to achieve uniform particle size distribution, high chemical purity, and lower interface energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) The high-purity cobalt sulfate obtained by recrystallizing reagent-grade cobalt sulfate is prepared into a cobalt sulfate solution with a concentration of 1mol / L by deionized water, and 0.02% polyvinyl alcohol and 2% ethylene glycol, stirred and mixed to A solution;
[0023] (2) Use reagent-grade ammonium oxalate, prepare an ammonium oxalate solution with a concentration of 0.5 mol / L with deionized water, add 2% reagent-grade ammonia water according to the weight ratio of the ammonium oxalate solution, stir and mix to form B solution;
[0024] (3) The molar ratio of cobalt sulfate and ammonium oxalate between solution A and solution B is 1:1, and solution A is sprayed at a flow rate of 500L / h and solution B at a flow rate of 1100L / h in parallel until an appropriate amount of deionization is added in advance The reaction was carried out in a water reactor, the reaction temperature was controlled at 25°C, the pH value of the solution during the reaction was 6.5-7.0, a...
Embodiment 2
[0027] (1) The high-purity cobalt nitrate obtained by recrystallizing reagent-grade cobalt nitrate is prepared into a cobalt nitrate solution with a concentration of 1mol / L by using deionized water, and 0.01% polypropylene alcohol and 5 % of acetone, stirred and mixed as A solution;
[0028] (2) Use reagent-grade ammonium oxalate, prepare an ammonium oxalate solution with a concentration of 0.3 mol / L with deionized water, add 0.2% reagent-grade ammonia water according to the weight ratio of the ammonium oxalate solution, stir and mix to form B solution;
[0029] (3) The molar ratio of cobalt nitrate and ammonium oxalate between solution A and solution B is 1:1.2, solution A is sprayed at a flow rate of 500L / h and solution B is at a flow rate of 1600L / h in parallel until an appropriate amount of deionization is added in advance The reaction is carried out in a water reactor, the reaction temperature is controlled at 35°C, the pH value of the solution during the reaction is 6.5 ...
Embodiment 3
[0032] (1) The high-purity cobalt chloride obtained after recrystallization of reagent-grade cobalt chloride is prepared into a cobalt chloride solution with a concentration of 0.5mol / L by using deionized water, and 0.01% of the cobalt chloride solution is added according to the weight ratio of the cobalt chloride solution. polyethylene glycol and 10% ethanol, stirred and mixed to be A solution;
[0033] (2) Use reagent-grade ammonium oxalate, prepare an ammonium oxalate solution with a concentration of 0.5 mol / L with deionized water, add 1% reagent-grade ammonia water according to the weight ratio of the ammonium oxalate solution, stir and mix to form B solution;
[0034] (3) The molar ratio of cobalt sulfate and ammonium oxalate between solution A and solution B is 1:1.4, solution A is sprayed at a flow rate of 1000L / h and solution B is at a flow rate of 1500L / h at a constant speed until an appropriate amount of deionization is added in advance The reaction is carried out in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com