Synthesis method of pentamethine cyanine dye for mercapto labeling
A technology of pentamethanine and a synthesis method, which is applied in the directions of methine/polymethine dyes, peptide preparation methods, organic dyes, etc. The problem of uneven end distribution, etc., achieves the effect of low toxicity, reducing background fluorescence interference, and improving signal-to-noise ratio.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
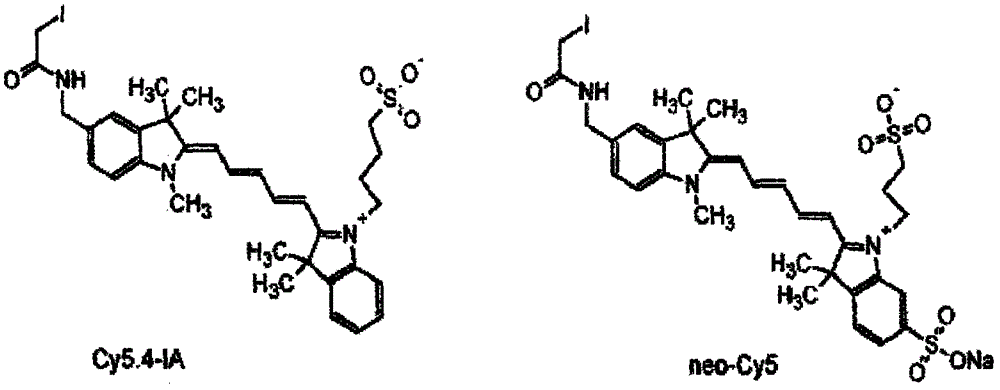
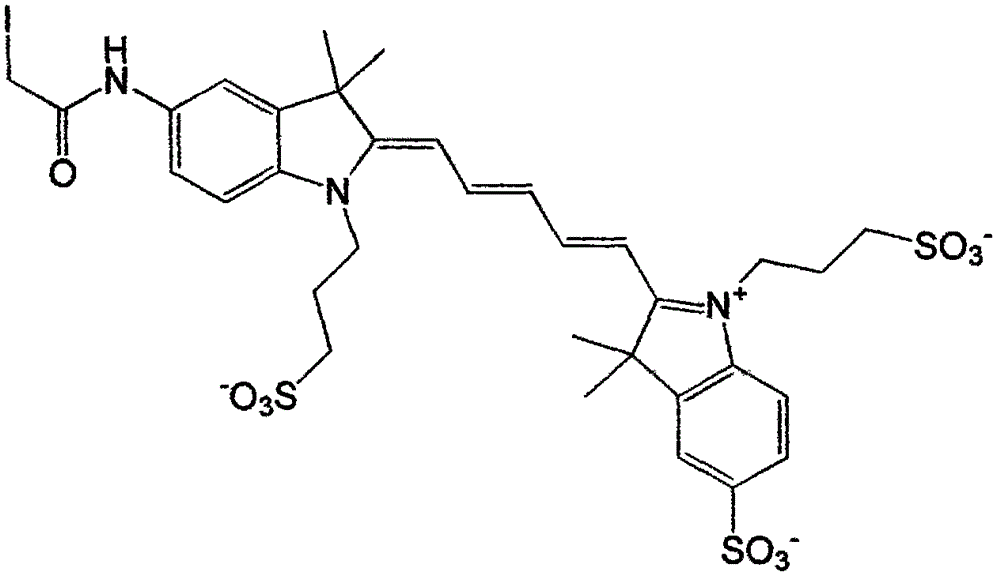
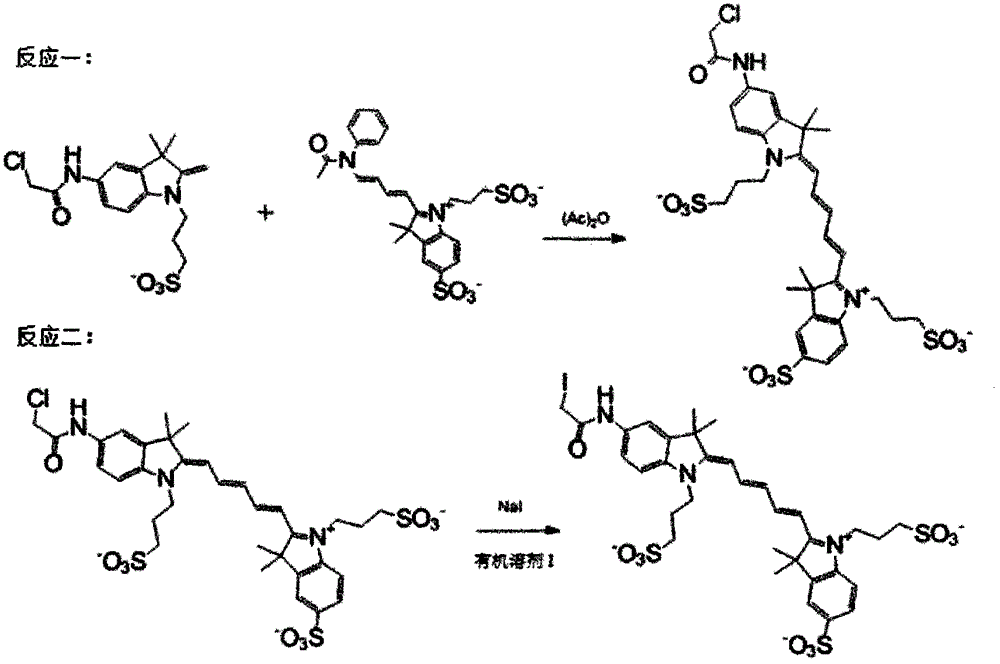
[0019] Specific implementation mode 1: see Figure 1-Figure 4 , this specific embodiment adopts the following technical scheme: its synthesis step is as follows:
[0020] The first step, reaction 1: take 1 equivalent of the potassium salt of compound I and 1.2 equivalent of the sodium salt of compound II, add 1.5 equivalents of chloroacetic anhydride, and stir at room temperature for 0.5 hours; rotary evaporation to remove the solvent, column chromatography, using methanol: Acetone=1:7 elution to obtain the corresponding compound III;
[0021] The second step, reaction 2: the obtained 1 equivalent of compound III and 1.1 equivalent of sodium iodide were reacted in a solvent of methanol:chloroform=1:1 at 60°C for 24 hours, filtered, and the solvent was removed by rotary evaporation to obtain the corresponding The final product IV; confirmed by nuclear magnetic spectrum is consistent with the target product.
[0022] In the structure of compound I, the corresponding cation is ...
specific Embodiment approach 2
[0026] Specific embodiment two: the difference between this specific embodiment and specific embodiment one is: its synthesis step is as follows:
[0027] The first step, reaction 1: take 1 equivalent of the sodium salt of compound I and 1.1 equivalent of the sodium salt of compound II, add 2 equivalents of chloroacetic anhydride, heat and stir for 0.6 hours; remove the solvent by rotary evaporation, column chromatography, and methanol : acetone=1:5 elution to obtain the corresponding compound III;
[0028] The second step, reaction 2: the obtained 1 equivalent of compound III and 1.5 equivalents of sodium iodide were reacted in a solvent of methanol:chloroform=1:2 at 70°C for 20 hours, filtered, and the solvent was removed by rotary evaporation to obtain the corresponding The final product IV; the NMR showed that it was consistent with the target product.
specific Embodiment approach 3
[0029] Specific embodiment three: the difference between this specific embodiment and specific embodiment one is: its synthesis step is as follows:
[0030] The first step, reaction one: take 0.40 g of the potassium salt of compound I and 0.62 g of the potassium salt of compound II, dissolve in 0.21 g of acetic acid, heat and stir for 1 hour; remove the solvent by rotary evaporation, column chromatography, use methanol: acetone =1:5 elution to obtain the corresponding compound III;
[0031] The second step, reaction two: the obtained 0.42g of compound III and 0.11g of sodium iodide were reacted in a solvent of methanol:chloroform=1:10 at 65°C for 24 hours, filtered, and the solvent was removed by rotary evaporation to obtain the corresponding The final product IV was 0.42 g; the NMR and mass spectrometry showed that it was consistent with the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com