Perfluorooctane-based compound and method for preparing perfluorooctane-containing terminated polyaryl ether sulphone
A technology for perfluorooctyl and polyaryl ether sulfone is applied in the field of preparing polyaryl ether sulfone containing perfluorooctane end groups and perfluorooctane compounds, and can solve the problems of reducing the friction coefficient of materials, high acquisition cost and preparation method. Cumbersome and other problems, to achieve the effect of increasing the water contact angle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
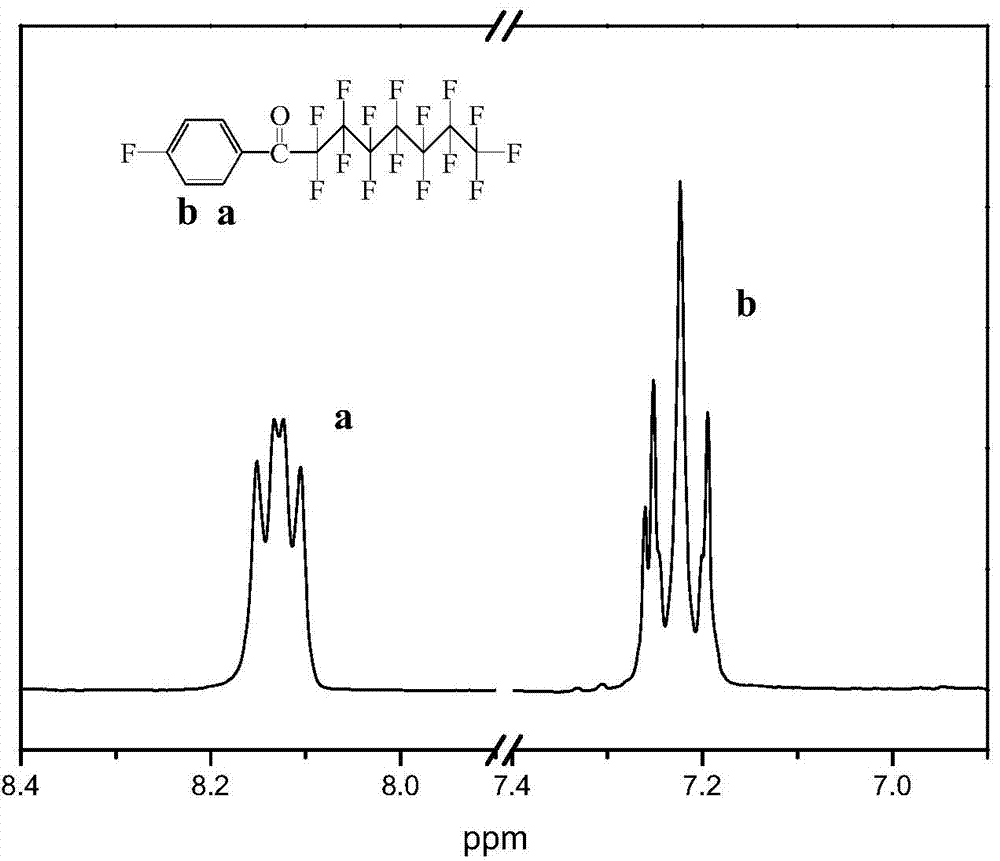
[0042] Add 14.67g of anhydrous aluminum chloride and 43mL of fluorobenzene into a single-necked flask equipped with a stirring device, start stirring, and slowly mix 43.25g of perfluorooctanoyl chloride and 50mL of fluorobenzene (a total of 93mL of fluorobenzene) at -10°C Put it into a single-necked bottle, install a calcium chloride drying tube at the mouth of the bottle to ensure the anhydrous environment of the system, stir at room temperature for 5 hours, pour the product into an aqueous HCl solution with a mass fraction of 1%, let it stand for liquid separation, and use the lower organic phase in sequence Weak alkaline water (pH 7.5-8.5), deionized water, and DMSO were washed twice each (stand still after each washing, and the lower organic phase was collected after layering to continue washing), and the finally obtained lower organic phase was distilled under reduced pressure. , to obtain about 40 g of a colorless liquid product, namely perfluorooctyl-(4-fluorophenyl) ket...
Embodiment 2
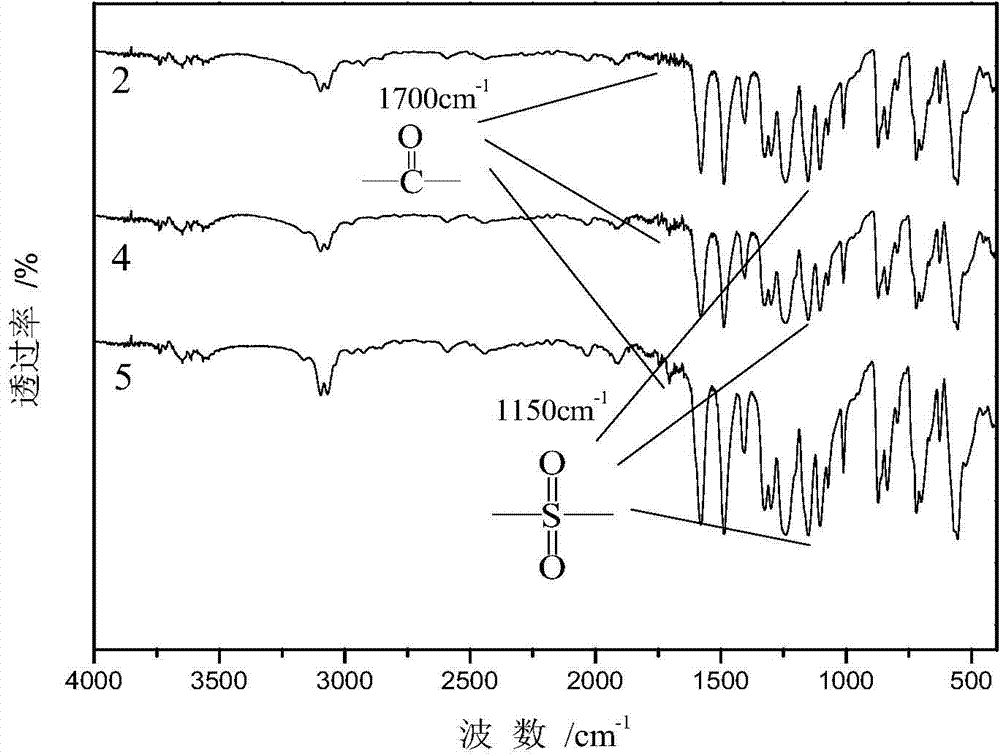
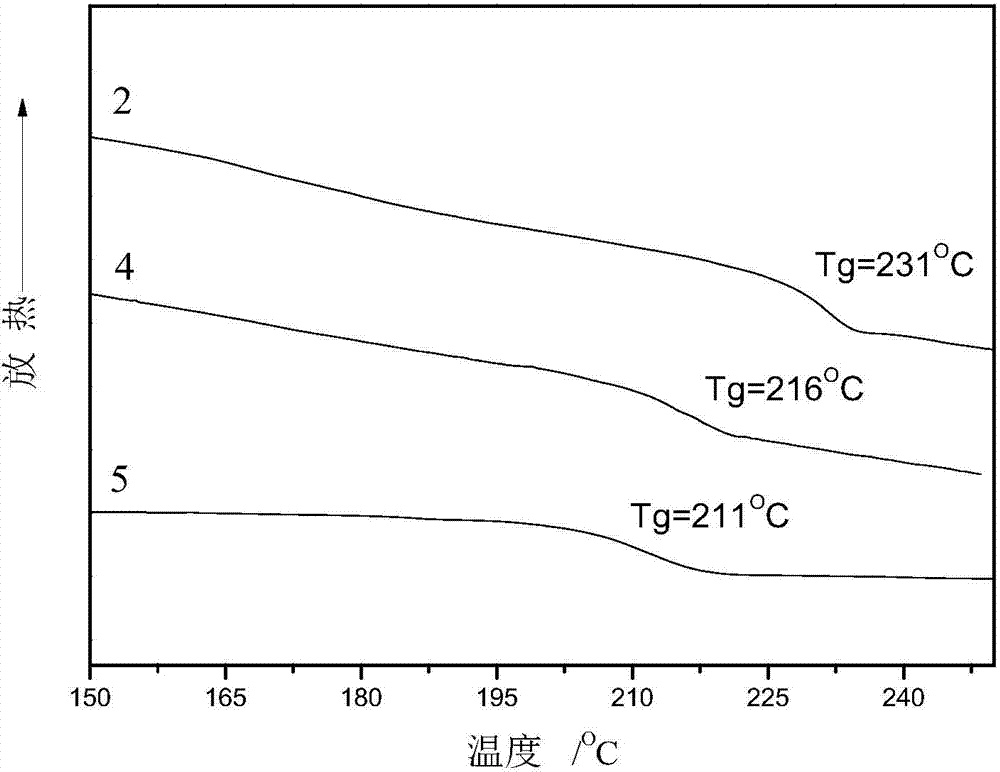
[0044] Add 6.36g 4,4'-difluorodiphenylsulfone and 6.39g 4,4'-dihydroxydiphenylsulfone to a three-necked flask equipped with a mechanical stirring device, a thermometer, a water heater, a condenser tube and a nitrogen protection device (102%), 2.97g anhydrous sodium carbonate, with 30mL sulfolane (TMS) as reaction solvent, solid content 25%, add 15mL toluene as water-carrying agent. The temperature of the system was raised to 140°C, 150°C, and 160°C with water for 1 hour respectively, the toluene was distilled off, the temperature was raised to 200°C for 3 hours, the temperature was lowered to 160°C, and 0.5 mL of capping agent perfluorooctyl-(4-fluorophenyl ) methyl ketone, the temperature was gradually raised to 200°C for 3 hours, the product was poured into deionized water, after crushing, it was washed with deionized water and absolute ethanol for 6 times, and the product was vacuum-dried at 80°C to obtain a white perfluorooctane end group Polyarylethersulfone powder, about...
Embodiment 3
[0046] Add 6.36g of 4,4'-difluorodiphenylsulfone and 6.51g of 4,4'-dihydroxydiphenylsulfone into a three-necked flask equipped with a mechanical stirring device, a thermometer, a water heater, a condenser tube and a nitrogen protection device (104%), 3.03g anhydrous sodium carbonate, with 30mL sulfolane (TMS) as reaction solvent, solid content 25%, add 15mL toluene as water-carrying agent. The temperature of the system was raised to 140°C, 150°C, and 160°C with water for 1 hour, and the toluene was evaporated, and the temperature was raised to 200°C for 3 hours. The product was vacuum-dried at 80° C. to obtain white hydroxyl-terminated polyarylethersulfone powder, about 9.52 g, with a yield of 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com