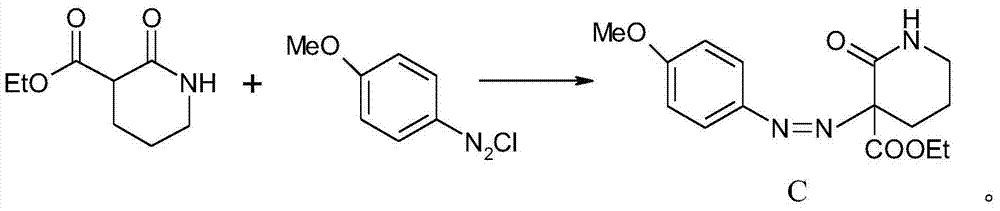
Synthesis method of melatonin
A synthesis method and technology of melatonin, applied in the direction of organic chemistry, etc., can solve the problems of low total yield, long steps, influence on promotion, etc., and achieve the effect of less solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
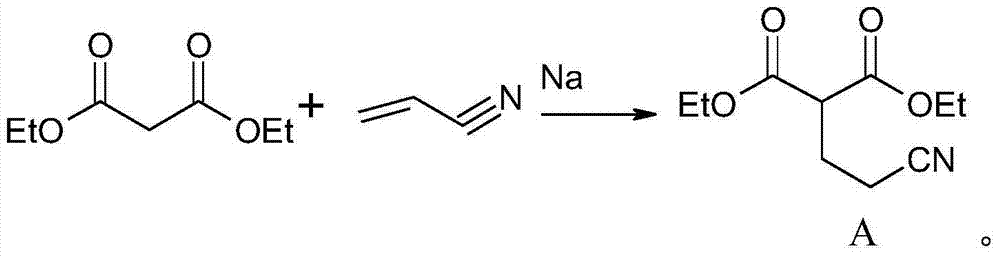
[0038] first step
[0039] Put 300Kg of diethyl malonate and 6Kg of sodium metal into a 500L reactor, add 100Kg of acrylonitrile dropwise below 80°C, and keep the reaction at 80°C for 2 hours after dropping. Cool down, add glacial acetic acid below 40°C to neutralize to pH 7. Transfer to the distillation pot, first recover diethyl malonate under reduced pressure, and when the top temperature reaches 100°C, add the adduct, then connect the fraction before 130°C to obtain 255Kg colorless transparent viscous liquid.
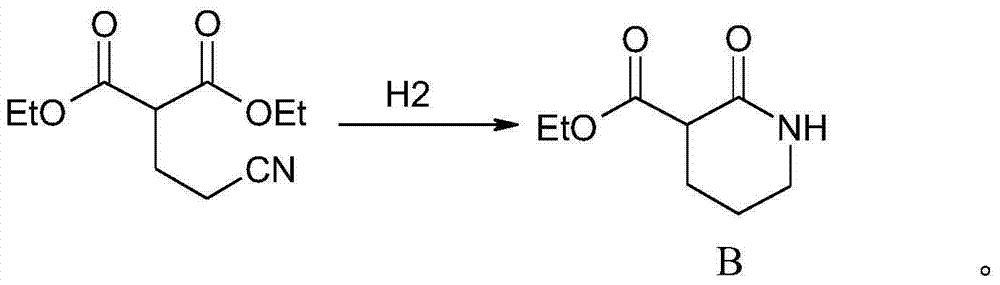
[0040] The second step: hydrogenation reaction
[0041] Add 100Kg of the adduct obtained in the previous step and 300L of absolute ethanol into a 500L autoclave, carefully add 24Kg of catalyst, raise the temperature to 35°C, vacuumize, pass hydrogen to 0.6MPa, and continue to pass hydrogen until the pressure drops. until the hydrogen is absorbed. Lower the temperature, let stand for more than 0.5 hours, pump the supernatant to the ethanol recovery kettle, and rec...
Embodiment 2
[0059] first step
[0060]Put 300Kg of diethyl malonate and 6Kg of sodium metal into a 500L reactor, add 100Kg of acrylonitrile dropwise below 80°C, and keep the reaction at 80°C for 2 hours after dropping. Cool down, add glacial acetic acid below 40°C to neutralize to pH 7. Transfer to the still, first recover diethyl malonate under reduced pressure, and when the top temperature is 100°C, add the adduct, then connect the fraction before 130°C to obtain 256Kg colorless transparent viscous liquid.
[0061] The second step: hydrogenation reaction
[0062] Add 100Kg of the adduct obtained in the previous step and 300L of absolute ethanol into a 500L autoclave, add 24Kg of catalyst, heat up to 30°C, vacuumize, and pass hydrogen until the pressure in the kettle reaches 0.5MPa, and then continue to pass hydrogen after the pressure drops , until no hydrogen is absorbed. Lower the temperature, let stand for more than 0.5 hours, pump the supernatant to the ethanol recovery kettle, a...
Embodiment 3
[0080] first step
[0081] Put 300Kg of diethyl malonate and 6Kg of sodium metal into a 500L reactor, add 100Kg of acrylonitrile dropwise below 80°C, and keep the reaction at 80°C for 2 hours after dropping. Cool down, add glacial acetic acid below 40°C to neutralize to pH 7. Transfer to the distillation pot, first recover diethyl malonate under reduced pressure, and when the top temperature reaches 100°C, add the adduct, then connect the distillate before 130°C to obtain 254Kg colorless transparent viscous liquid.
[0082] The second step: hydrogenation reaction
[0083] Add 100Kg of the adduct obtained in the previous step and 300L of absolute ethanol into a 500L autoclave, carefully add 24Kg of catalyst, raise the temperature to 35°C, vacuumize, and pass hydrogen to 0.5MPa. After the pressure drops, continue to pass hydrogen until it stops. until the hydrogen is absorbed. Cool down, let it stand for more than 0.5 hours, pump the supernatant liquid into an ethanol recover...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com