A kind of method for synthesizing cyclohexylbenzene by liquid-phase alkylation of benzene and cyclohexene
A technology for liquid-phase alkylation of cyclohexene and cyclohexylbenzene is applied in the field of liquid-phase alkylation of benzene and cyclohexene to synthesize cyclohexylbenzene, and can solve the problems of unsatisfactory, easy deactivation, and high corrosiveness of catalysts, etc. Achieve the effect of prolonging operating life and inhibiting deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
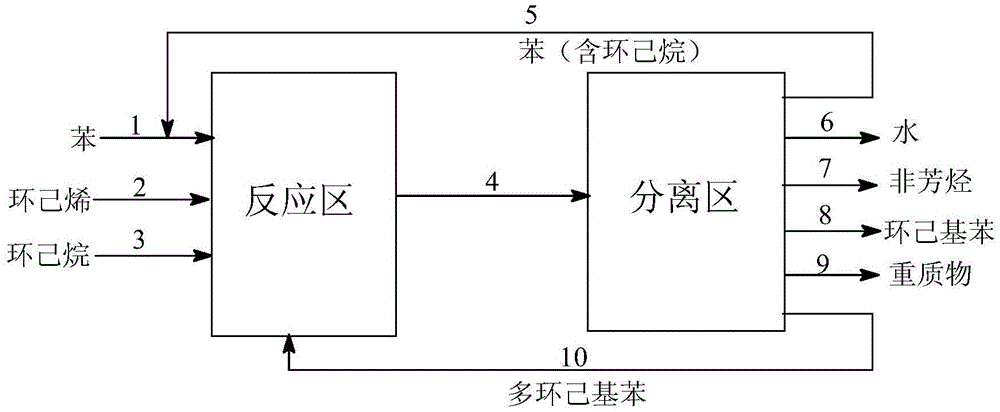
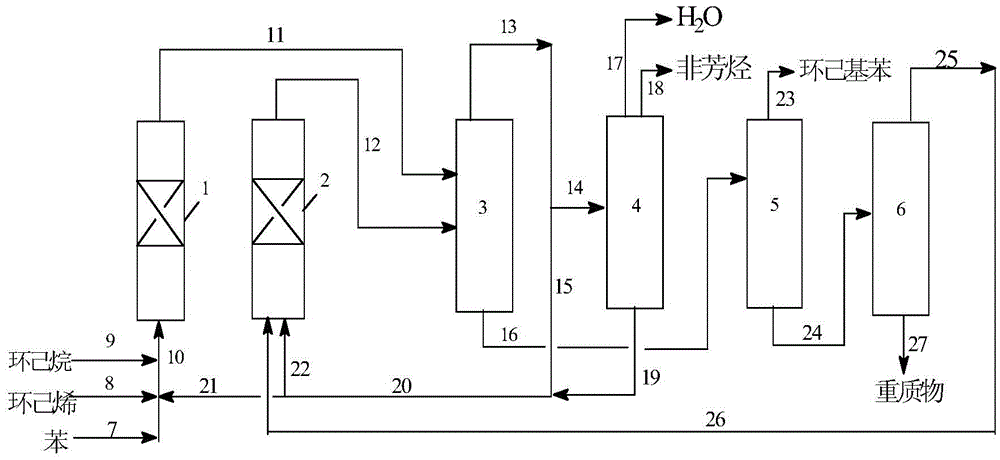
[0045] In the fixed-bed reactor of Process I shown in the figure, the catalyst containing Y-type molecular sieves is loaded, and the upper and lower parts of the reactor are filled with porcelain balls respectively. First, continuously enter benzene into the reactor, raise the temperature to 150°C, and control the pressure at 3.0Mpa. -1 Under the condition, the raw materials are added to the reactor according to the benzene / cyclohexene molar ratio of 12:1 for continuous reaction. Take the sample of the top effluent of the reactor and analyze the product composition with Shimadzu 2010 gas chromatography equipped with 50m×0.25mm capillary column and FID detector. The conversion rate of cyclohexene reaches 100%, and the selectivity of cyclohexylbenzene can reach 93.8 %, the selectivity of cyclohexylation is 99.2, and the life of the catalyst is 985h (the time when the conversion rate of cyclohexene is greater than 99.8%, the same below). The reaction product enters the separatio...
Embodiment 2
[0049] In the fixed-bed reactor of Process I shown in the figure, the catalyst containing Y-type molecular sieves is loaded, and the upper and lower parts of the reactor are filled with porcelain balls respectively. First, continuously enter benzene into the reactor, raise the temperature to 150°C, and control the pressure at 3.0Mpa. After stabilization, use a plunger pump under the condition of liquid hourly space velocity 2h-1, according to the molar ratio of benzene / cyclohexene / cyclohexane 12:1:1 Add raw materials to the reactor for continuous reaction. Take the sample of the top effluent of the reactor and analyze the product composition with Shimadzu 2010 gas chromatography equipped with 50m×0.25mm capillary column and FID detector. The conversion rate of cyclohexene reaches 100%, and the selectivity of cyclohexylbenzene can reach 92.9% %, the cyclohexylation selectivity is 98.5%, and the lifetime of the catalyst is 1805h. The reaction product enters the separation zone ...
Embodiment 3
[0053] In the fixed-bed reactor of Process I shown in the figure, the catalyst containing Y-type molecular sieves is loaded, and the upper and lower parts of the reactor are filled with porcelain balls respectively. First, continuously enter benzene into the reactor, raise the temperature to 150°C, and control the pressure at 3.0Mpa. -1 Under the condition, the raw materials are added into the reactor according to the molar ratio of benzene / cyclohexene / cyclohexane 12:1:2 for continuous reaction. The sample of the effluent from the top of the reactor was analyzed by a Shimadzu 2010 gas chromatograph equipped with a 50m×0.25mm capillary column and an FID detector to analyze the composition of the product. The conversion rate of cyclohexene reached 100%, and the selectivity of cyclohexylbenzene could reach 91.7 %, the cyclohexylation selectivity is 98.4%, and the lifetime of the catalyst is 2060h. The reaction product enters the separation zone to separate benzene, cyclohexane, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com