Resveratrol high-molecular bonding drug and preparation method thereof
A technology of resveratrol and high-molecular polymer, applied in the field of medicine, can solve the problems of large influence of inclusion effect, no water solubility and stability involved, etc., achieve good hydrophilicity and biocompatibility, avoid The effect of phagocytosis and efficient targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
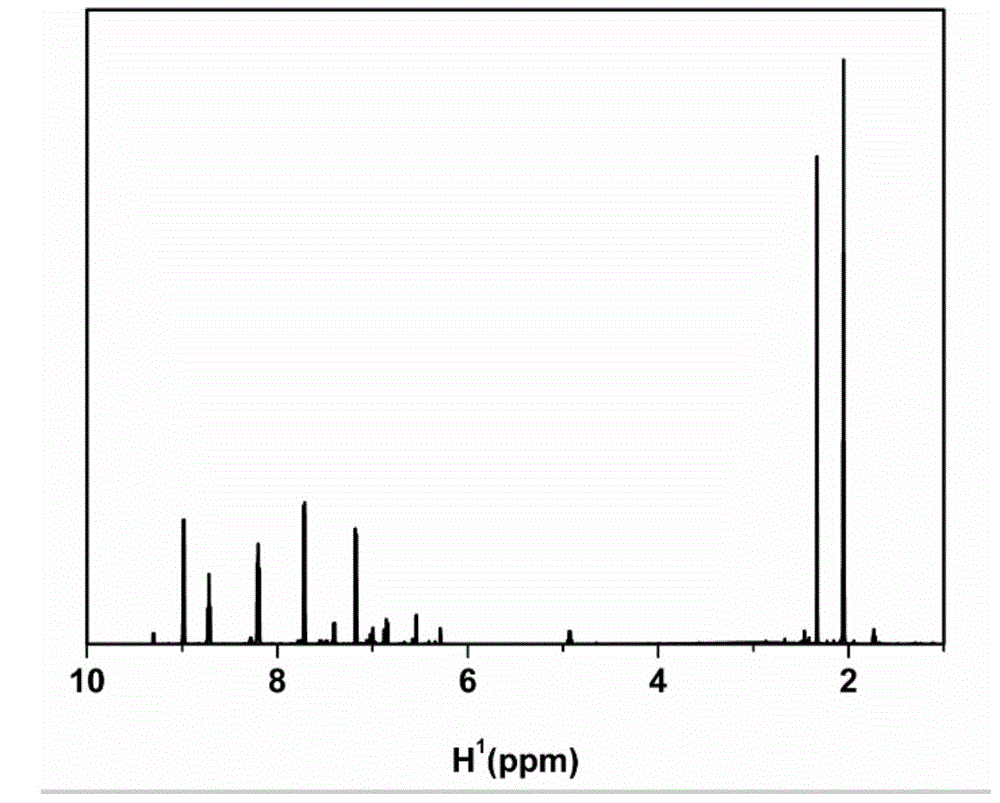
[0060] Add 1.9g of p-toluenesulfonyl chloride into a dry reactor with a water separation device and a stirring device, replace the argon gas 3 times, azeotropically remove water with anhydrous toluene, remove most of the toluene, and change it to a decompression device to extract Toluene remained and was vacuum dried at 80°C for 6 hours. After returning to room temperature, add 3.00 g of recrystallized resveratrol and 100 ml of anhydrous acetone under the protection of an inert gas, and add a small amount of NaHCO to the reaction at the same time 3 , and reacted at 25°C for 18 hours under magnetic stirring. The product was filtered to remove NaHCO 3 , the supernatant was suspended and evaporated to a molten state to remove a large amount of acetone, the product was dissolved in appropriate dichloromethane, settled in ether, and dried in vacuo to obtain 4.10 g of a white powder product with a yield of 83.7%.
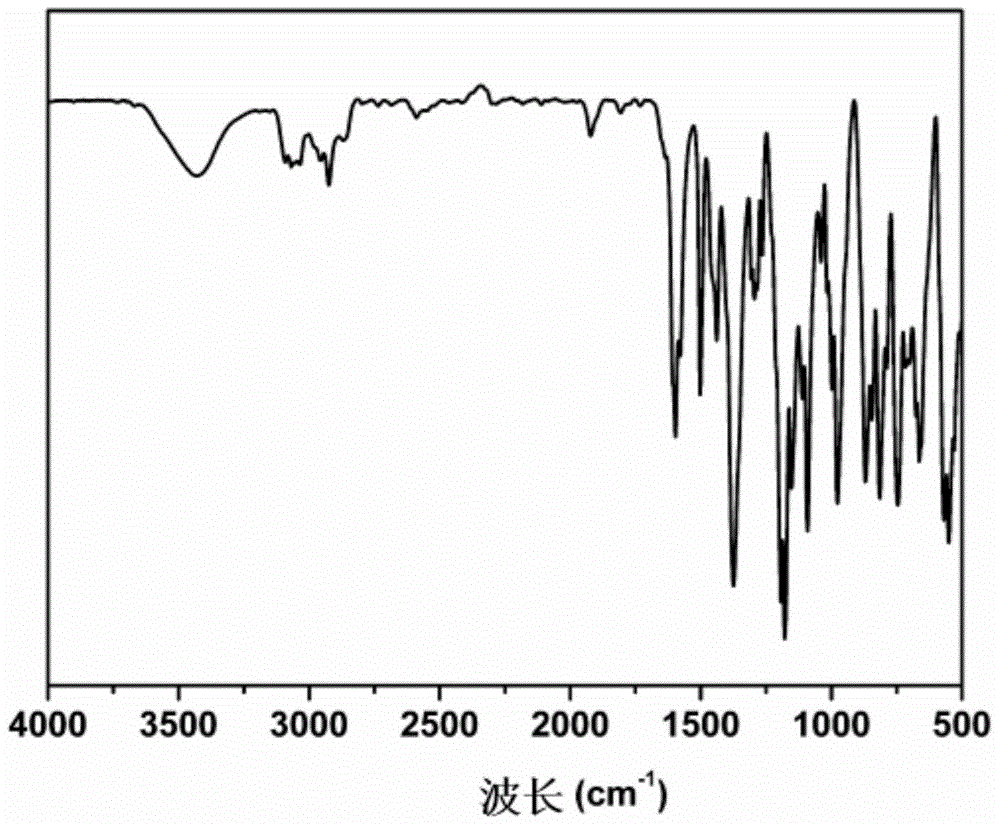
[0061] The infrared spectrum of gained resveratrol derivative sees...
Embodiment 2
[0065] Add 1.90g of p-toluenesulfonyl chloride into a dry reactor with a water separation device and a stirring device, replace the argon gas 3 times, azeotropically remove water with anhydrous toluene, remove most of the toluene, and change to a decompression device to extract Toluene remained and was vacuum dried at 80°C for 6 hours. After returning to room temperature, 3.00 g of recrystallized resveratrol and 100 ml of anhydrous acetone were added under the protection of an inert gas, and a small amount of NaHCO was added to the reaction 3 , and reacted at 25°C for 18 hours under magnetic stirring. The product was filtered to remove NaHCO 3 , the supernatant was suspended and evaporated to a molten state to remove a large amount of acetone, the product was dissolved in appropriate dichloromethane, settled in ether, and dried in vacuo to obtain 4.10 g of a white powder product with a yield of 83.7%.
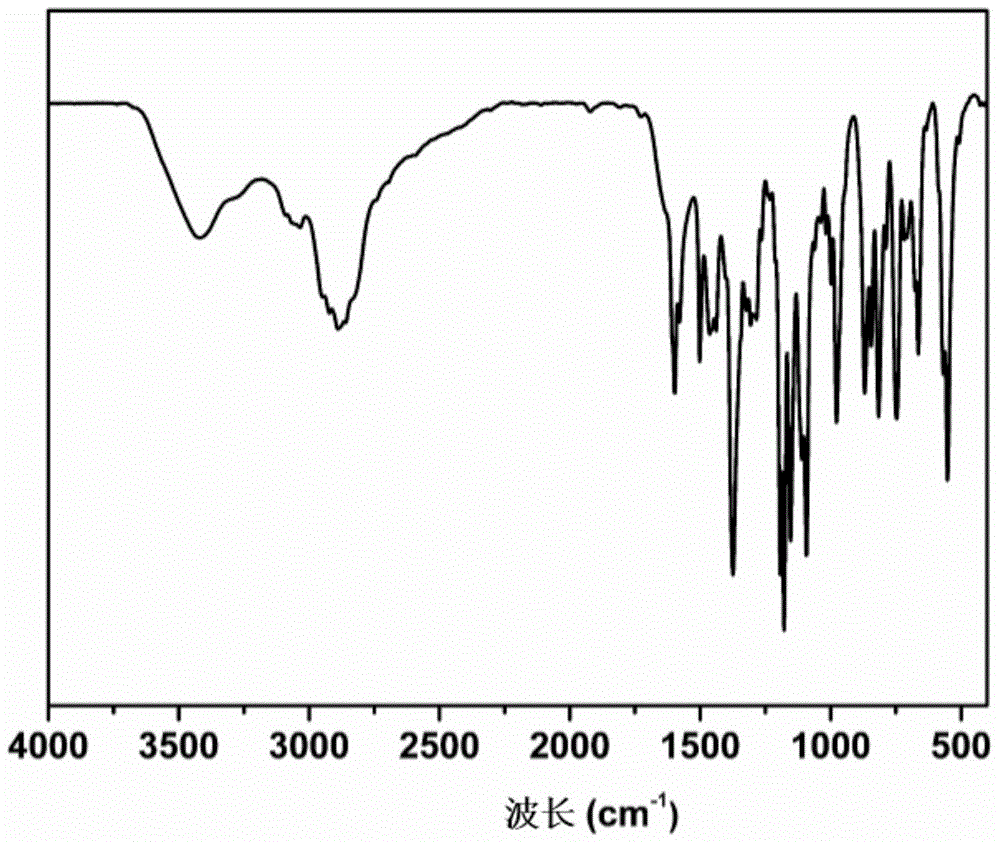
[0066] The infrared test results of the obtained synthetic product show ...
Embodiment 3
[0069] Add 785 mg of acetyl chloride into a dry reactor with a water separator and a stirring device, replace the argon gas 3 times, soak the molecular sieve to remove water, remove most of the water, and change to a decompression device to extract the purified acetyl chloride. Add to 100ml of anhydrous acetone under the protection of inert gas and add recrystallized resveratrol 3.00g, add a small amount of NaHCO to the reaction at the same time 3 , and reacted at 25°C for 20 hours under magnetic stirring. The product was filtered to remove NaHCO 3 , the supernatant was suspended and evaporated to a molten state to remove a large amount of acetone, and the product was dissolved in appropriate dichloromethane, settled with ether, and dried in vacuo to obtain 2.80 g of a white powder product with a yield of 74.0%.
[0070] The infrared test results of the obtained synthetic product show that the product contains both the resveratrol structure and the characteristic absorption p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com