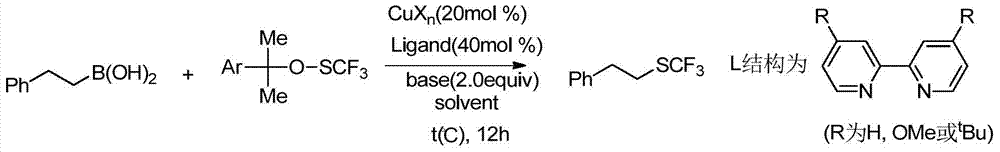
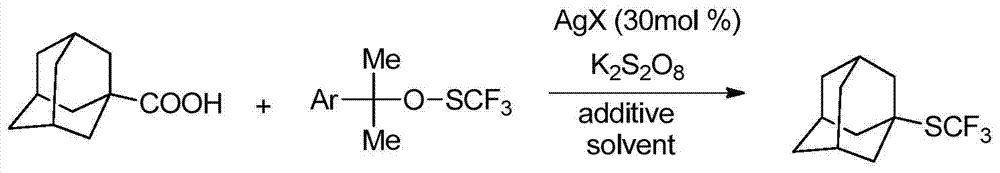
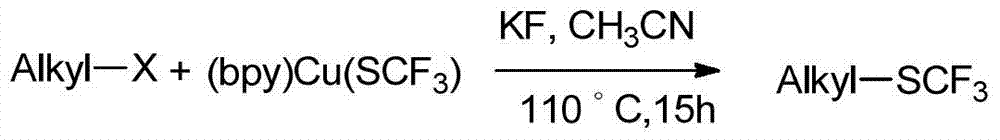
A kind of alkyl trifluoromethyl sulfide compound and preparation method thereof
A technology of alkyl trifluoromethyl sulfide and trifluoromethyl sulfide, which is applied in the field of alkyl trifluoromethyl sulfide compounds and their preparation, and can solve the problem that trifluoromethyl sulfide reagents are difficult to obtain and are not suitable for industrial production. , harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] Example 1 C-H direct trifluoromethylthiolation reaction of cyclooctane, silver trifluoromethylthio and potassium persulfate
[0049]
[0050] Add silver trifluoromethylsulfanyl (0.5mmol), N-hydroxyphthalimide (0.1mmol) and potassium persulfate (1.0mmol) into the reaction flask, add cyclooctane (1.0mmol) after pumping argon three times mmol) and acetonitrile (5 mL). The reaction bottle was placed in an oil bath at 80°C and started to heat and stir for 4 hours, then took it out, and cooled to room temperature. With benzotrifluoride as the internal standard substance, using 19 F-NMR confirmed the formation of cyclooctyl trifluoromethyl sulfide (yield 82%). The reaction solution was filtered, and the filtrate was used to remove the solvent by a rotary evaporator, and separated by bottle-neck-bottle distillation to obtain a colorless oily liquid (64 mg, yield 60%). The relevant data are as follows: 1 H NMR (400MHz, CDCl 3 ): δ3.51-3.44(m,1H),2.09-2.02(m,2H),1.80-1.71...
Embodiment 2
[0051] Example 2 The decarboxylation trifluoromethylthiolation of cyclohexanecarboxylic acid, silver trifluoromethylthio and potassium persulfate
[0052]
[0053]Silver trifluoromethylthio (1.5mmol), pyridine (0.1mmol) and potassium persulfate (4.5mmol) were added to the reaction flask, and cyclohexanecarboxylic acid (1.0mmol) and acetonitrile (20mL) were added after argon was pumped three times. Place the sealed tube in an oil bath at 60°C and start heating and stirring for 24 hours, then take it out, and let it cool to room temperature. With benzotrifluoride as the internal standard substance, using 19 F-NMR confirmed the formation of cyclohexyltrifluoromethylsulfide (83% yield). Formation of this product was also confirmed by GC-MS. The relevant data are as follows: m / z=184 (GC-MS; EI).HRMS-EI: Calculated for C 7 h 11 f 3 S:184.0534,Found:184.0535.Crude 1 H NMR (400MHz, CD 3 CN): δ3.35(tt,J=4Hz,1H),2.06-1.27(m,10H)ppm.Crude 19 F NMR (376MHz, CD 3 CN):δ-39.78(s,...
Embodiment 3
[0054] Coupling reaction of embodiment 3 cyclodecyl boronic acid, silver trifluoromethylthio and potassium persulfate
[0055]
[0056] Add silver trifluoromethylthio (0.5mmol), o-phenanthroline (0.5mmol) and potassium persulfate (1.0mmol) into the reaction flask, add cyclodecylboronic acid (3.0mmol) and Acetonitrile (15 mL). Place the sealed tube in an oil bath at 25°C and start heating and stirring for 36 hours, then take it out, and wait to cool to room temperature. With benzotrifluoride as the internal standard substance, using 19 It was confirmed by F-NMR that cyclodecyltrifluoromethylsulfide was produced (yield: 74%). The reaction solution was filtered, and the filtrate was used to remove the solvent by a rotary evaporator, and separated by bottle-neck-bottle distillation to obtain a colorless oily liquid (65 mg, yield 55%). The relevant data are as follows: 1 H NMR (400MHz, CDCl 3 ):δ3.58-3.51(m, characteristic CH-SCF 3 ),1.97-1.79(m,4H),1.65-1.63(m,4H).1.54-1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com