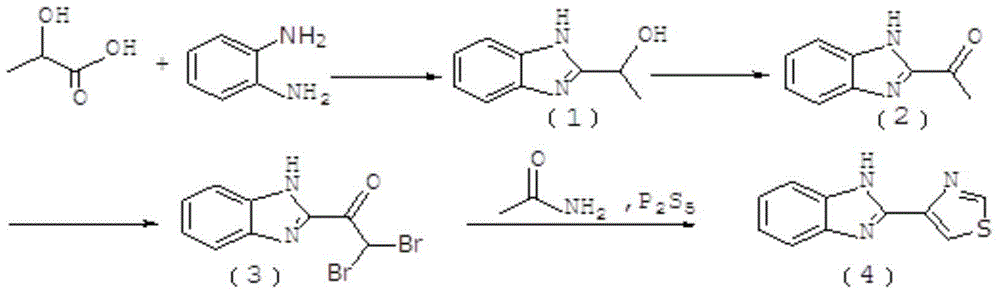
New method for preparing thiabendazole
A technology of thiabendazole and thiazole, which is applied in the field of drug synthesis, can solve the problems of high reaction temperature, low yield, unfavorable environmental protection, etc., and achieve the effect of high atom utilization rate and beneficial to green chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
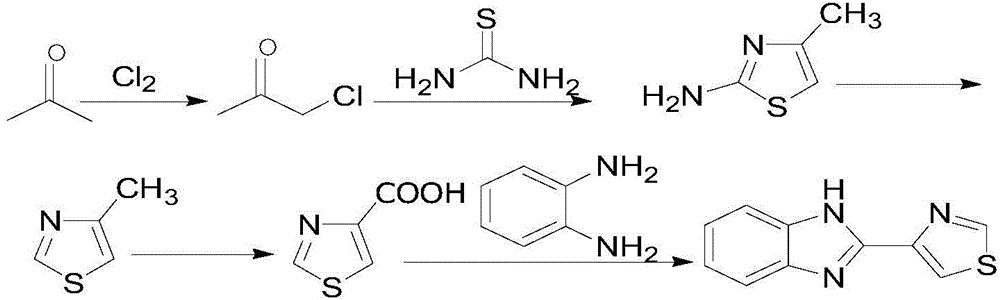
[0056] 1.1) Preparation of monochloroacetone
[0057] Add 200g of acetone, 20g of calcium carbonate and 10mL of water into a 500mL four-necked bottle, connect the rectification column, and connect the chlorination reactor and condenser at the upper port of the column; start to heat up and reflux, pass chlorine gas, and stop the chlorine flow when the temperature rises to 100°C , to end the reaction. The reactants contained 1.8% of acetone, 95.4% of monochloroacetone, 1.2% of 1,1-dichloroacetone and 1.6% of other impurities according to gas phase detection.
[0058] 1.2) Synthesis of 2-amino-4-methylthiazole
[0059] Add 113.88 g (1.50 mol) of thiourea directly to the monochloroacetone reaction solution (containing 125 g of monochloroacetone, 1.36 mol), stir to form a suspension, and heat to reflux in a water bath for 5 h. The reaction solution was poured into a beaker containing 500 g of ice-water mixture, cooled in a cryopump, and then 200 g of sodium hydroxide was added wi...
Embodiment 2
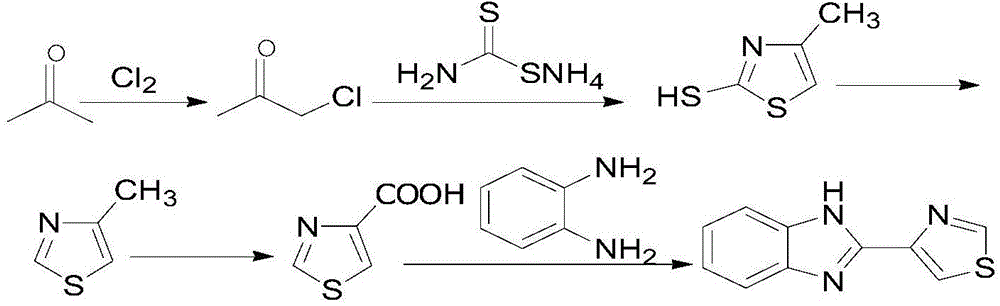
[0069] 2.1) Preparation of monochloroacetone
[0070] Add 200g of acetone and 10mL of water into a 500mL four-necked flask, raise the temperature and reflux to flow chlorine, slowly add 20% aqueous sodium hydroxide solution into the reaction flask to make the pH of the feed solution = 3-4, and stop the flow when the temperature rises to 100°C. Chlorine ends the reaction. The reactants contained 2.0% of acetone, 94.2% of monochloroacetone, 1.5% of 1,1-dichloroacetone and 2.3% of other impurities according to gas phase detection.
[0071] 2.2) Synthesis of 2-mercapto-4-methylthiazole
[0072] Mix monochloroacetone (74.0g, 0.8mol) with 200mL of water, adjust the pH to 2 with dilute hydrochloric acid, cool the mixture to 40°C, then add a solution of ammonium dithiocarbamate (132g, 1.2mol) and 50mL of water , the reaction mixture was stirred at 40°C for 5 h, and after the reaction was stopped, the pH was adjusted to 9 with sodium hydroxide, extracted with 3*100 mL of ethyl acetat...
Embodiment 3
[0082] 3.1) Preparation of monochloroacetone
[0083] Add 180g of acetone, 30g of calcium carbonate and 10mL of water into a 500mL four-necked bottle, connect the rectification column, and connect the chlorination reactor and condenser at the upper port of the column; start to heat up and reflux, pass chlorine gas, and stop the chlorine flow when the temperature rises to 110°C , to end the reaction. The gas phase detection of the reactant contained 2.1% of acetone, 95.6% of monochloroacetone, 1.4% of 1,1-dichloroacetone, and 0.9% of other impurities.
[0084] 3.2) Synthesis of 2-amino-4-methylthiazole
[0085] Add 113.88 g (1.50 mol) of thiourea directly to the reaction liquid of chloroacetone (containing 115 g of 1.25 mol of chloroacetone), stir to form a suspension, and heat to reflux in a water bath for 6 hours. The reaction solution was poured into a beaker containing 480g of ice-water mixture, cooled in a cryopump, and then 180g of sodium hydroxide was added with stirri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com