Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cefalexin, cefadroxil and cefradine
An enzyme-linked immunosorbent reagent, cefadroxil technology, applied in the field of drug residue analysis and immunology, can solve the problems of lack of specificity of methods, differences in sensitivity of antimicrobial drugs, expensive instruments, etc., and achieve simple sample processing methods and harm to health. Small, time and cost saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
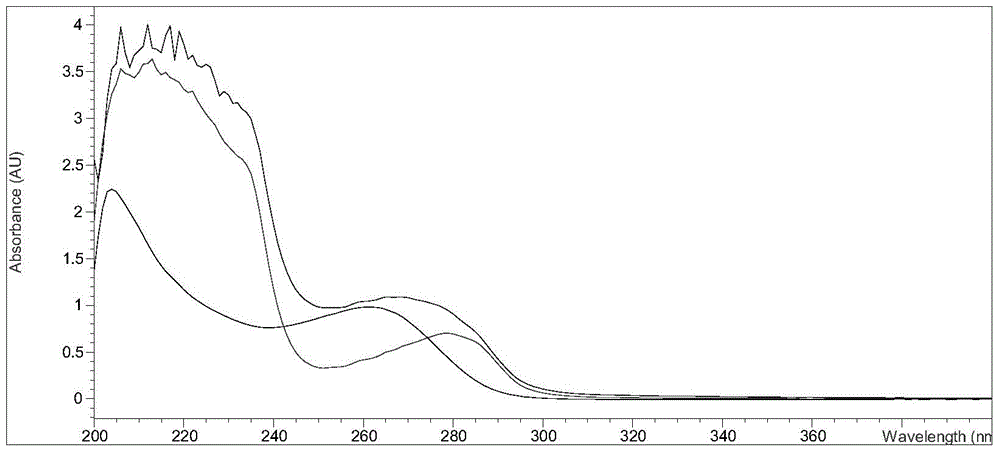
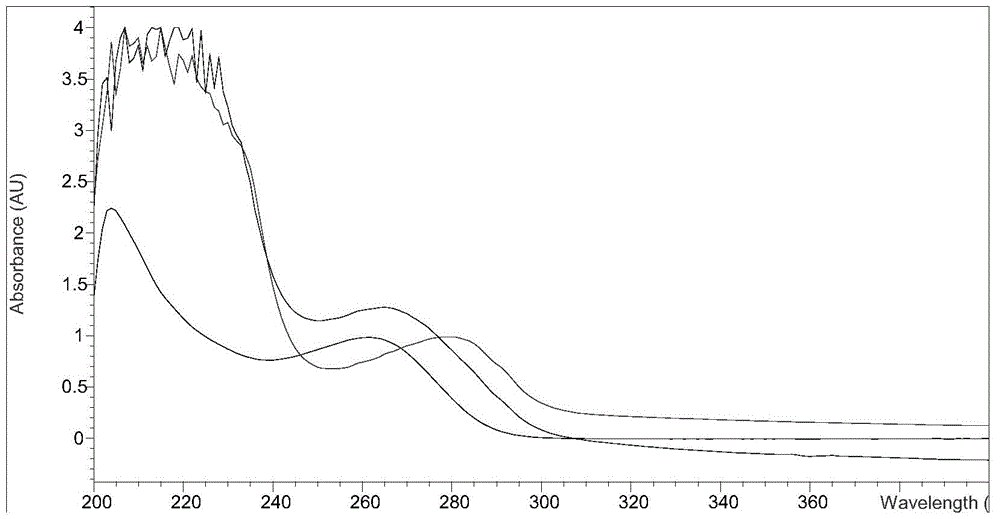
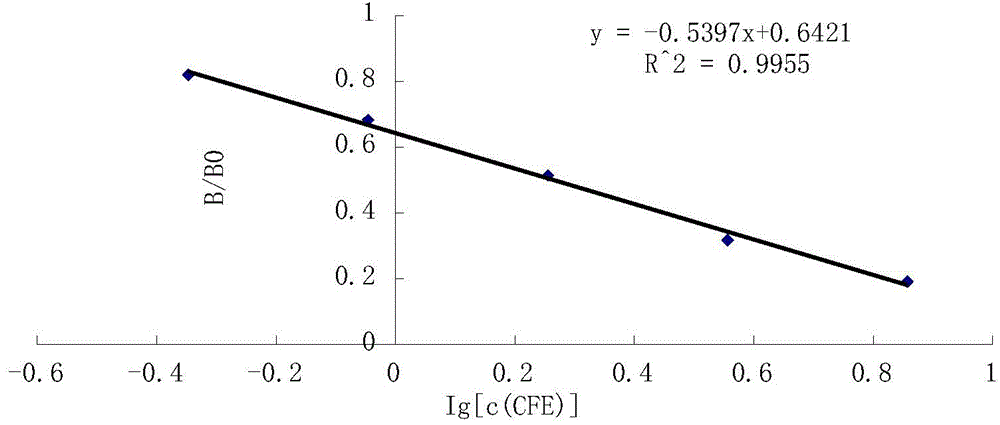
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 immunogen and coating former
[0031] 1.1 Preparation of immunogen
[0032] Accurately weigh 36.5 mg of cephalexin and completely dissolve it in 4 mL of PBS to obtain solution A. Accurately weigh 68mg of BSA and fully dissolve it in 6mL of PBS to form B solution. Another 120.0 mg of carbodiimide (EDC) and 60 mg of N-hydroxysuccinimide (NHS) were dissolved in 1 mL of LPBS to obtain solution C. In ice bath, add liquid C dropwise to liquid B under magnetic stirring, react in ice bath for 4h, then add the mixed solution dropwise into liquid A, and react in ice bath for 12h. After the reaction is completed, transfer the reaction solution into a dialysis bag, dialyze in PBS at 4°C for 3 days, change the dialysate every 4 to 6 hours, and freeze-dry the sample after the dialysis to obtain the conjugate cephalexin-BSA, and place it at -20 Store at ℃.
[0033] 1.2 Preparation of coating agent
[0034] Dissolve 120 mg of OVA in 8 mL of phosphat...
Embodiment 2
[0035] The preparation of embodiment 2 monoclonal antibody
[0036] Preparation of hybridoma cells: referring to "Animal Immunology" by Yang Hanchun, Balb / C mice (purchased from the Experimental Animal Center of Hubei Academy of Medical Sciences) were immunized with the immunogen cephalexin-bovine serum albumin conjugate prepared in Example 1. The immunization procedure is as follows: for basic immunization, a protein emulsion containing 100 μg of immunogen emulsified with an equal volume of Freund’s complete adjuvant is injected subcutaneously at the back of the neck of the mouse; Protein emulsion of immunogen for booster immunization. From the third immunization, the tail blood was collected on the 8th day after each immunization, the serum was separated, and the serum antibody titer was detected by indirect ELISA method. Immunization qualified mice (high titer, good sensitivity) stop immunization in preparation for fusion. At the time of fusion, take a Balb / C mouse that h...
Embodiment 3
[0041] The establishment of embodiment 3 indirect competition ELISA detection method
[0042] 3.1 Preparation of reagents (the reagents used in this example were prepared by the following methods unless otherwise specified)
[0043] Phosphate buffer: NaCl 8.0g, KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 O 2.9g, KCl 0.2g, add double distilled water to 1000mL, adjust pH to 7.4;
[0044] Coating solution: Take Na 2 CO 3 1.59g, NaHCO 3 2.93g, add triple distilled water to 1000mL, adjust the pH value to 9.6;
[0045] Washing liquid: NaCl 8.0g, KH 2 PO 4 0.2g, Na 2 HPO 4 12H 2 O 2.9g, KCl 0.2g, Tween 20 0.5mL, add double distilled water to 1000mL, adjust pH to 7.4;
[0046] Blocking solution: Ovalbumin 1g dissolved in 100mL phosphate buffer;
[0047] Substrate solution A: 3,3',5',5-tetramethylbenzidinediamine (TMB) 200mg, absolute ethanol 100mL, add double distilled water to 1000mL;
[0048] Substrate B: Na 2 HPO 4 14.6g, citric acid 9.3g, 0.75% urea hydrogen peroxide 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com