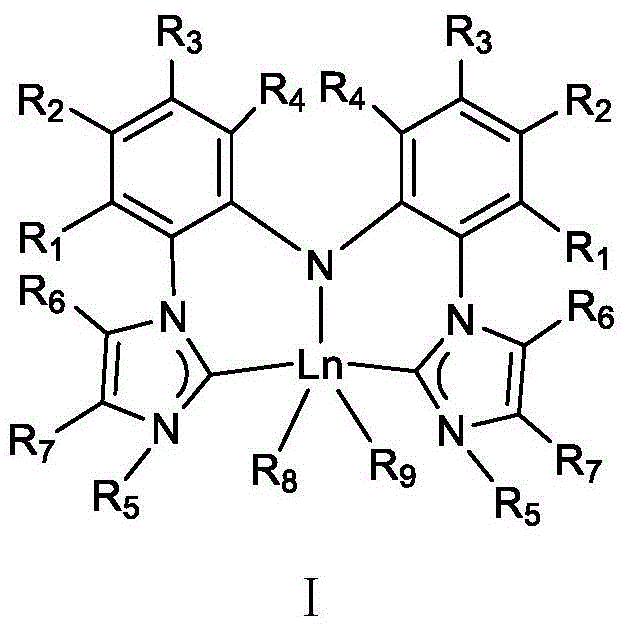
Pincerlike bi-N-heterocyclic carbene biphenyl amine rare earth metal catalyst, preparation method and application thereof
A technology of heterocyclic carbene and bisphenylamine, which is applied in the field of catalysts and can solve the problems that the preparation method of pincer bis-N-heterocyclic carbene bisphenylamine rare earth metal catalyst has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Preparation of clamp-shaped bis-N-heterocyclic carbene bisphenylamine ligand
[0065]
[0066] First, under a nitrogen atmosphere, 8.65 g (30.58 mmol) of o-iodobromobenzene, 5.26 g (30.58 mmol) of 2-bromoaniline, 4.11 g (42.81 mmol) of sodium n-butoxide, 0.03 g (0.15 mmol) of palladium acetate, bis Add 0.12 g (0.23 mmol) of (2-diphenylphosphine) phenyl ether into 100 ml Schlenk bottles, dissolve in 20 ml toluene to obtain mixture a; heat to 110°C in the dark, take it out after 12 hours of reaction, cool to 25°C, Add saturated brine to the mixture to obtain an organic phase, add anhydrous magnesium sulfate (5g), filter, take the filtrate and carry out rotary evaporation to obtain a mixture b containing the first step product; column chromatography obtains the first step product c, the yield 94%; secondly, the first step product c 2.97g (9.10mmol), imidazole 1.24g (18.2mmol), cesium carbonate 11.86g (36.40mmol), cuprous iodide 0.35g (1.82mmol) were added to 100ml ...
Embodiment 2
[0071] (1) Preparation of clamp-shaped bis-N-heterocyclic carbene bisphenylamine ligand
[0072]
[0073] First, under a nitrogen atmosphere, 8.65 g (30.58 mmol) of o-iodobromobenzene, 5.26 g (30.58 mmol) of 2-bromoaniline, 4.11 g (42.81 mmol) of sodium n-butoxide, 0.03 g (0.15 mmol) of palladium acetate, bis Add 0.12 g (0.23 mmol) of (2-diphenylphosphine) phenyl ether into 100 ml Schlenk bottles and dissolve in 20 ml toluene to obtain mixture a; heat to 120°C in the dark, take it out after 16 hours of reaction, and cool to 25°C. Add saturated brine to the mixture to obtain an organic phase, add anhydrous magnesium sulfate (10g), filter, take the filtrate and carry out rotary evaporation to obtain a mixture b containing the product of the first step; column chromatography obtains the product c of the first step, and the yield 94%; secondly, the first step product c 2.97g (9.10mmol), imidazole 1.24g (18.2mmol), cesium carbonate 11.86g (36.40mmol), cuprous iodide 0.35g (1.82m...
Embodiment 3
[0078] (1) Preparation of clamp-shaped bis-N-heterocyclic carbene bisphenylamine ligand
[0079]
[0080] First, under a nitrogen atmosphere, 8.65 g (30.58 mmol) of o-iodobromobenzene, 5.26 g (30.58 mmol) of 2-bromoaniline, 4.11 g (42.81 mmol) of sodium n-butoxide, 0.03 g (0.15 mmol) of palladium acetate, bis Add 0.12g (0.23mmol) of (2-diphenylphosphine)phenyl ether into 100ml Schlenk bottles, dissolve in 20ml toluene to obtain mixture a; heat to 115°C in the dark, take it out after 14h of reaction, cool to 25°C, Add saturated brine to the mixture to obtain an organic phase, add anhydrous magnesium sulfate (8g), filter, take the filtrate and carry out rotary evaporation to obtain a mixture b containing the first step product; column chromatography obtains the first step product c, the yield 94%; secondly, the first step product c 2.97g (9.10mmol), imidazole 1.24g (18.2mmol), cesium carbonate 11.86g (36.40mmol), cuprous iodide 0.35g (1.82mmol) were added to 100ml In a round...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com