A kind of preparation method of ring brush polymer containing polystyrene main chain
A technology of polystyrene and linear polystyrene, which is applied in the field of preparation of ring-brush polymers, can solve problems such as difficult to achieve, and achieve the effects of fast reaction rate, high yield, and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
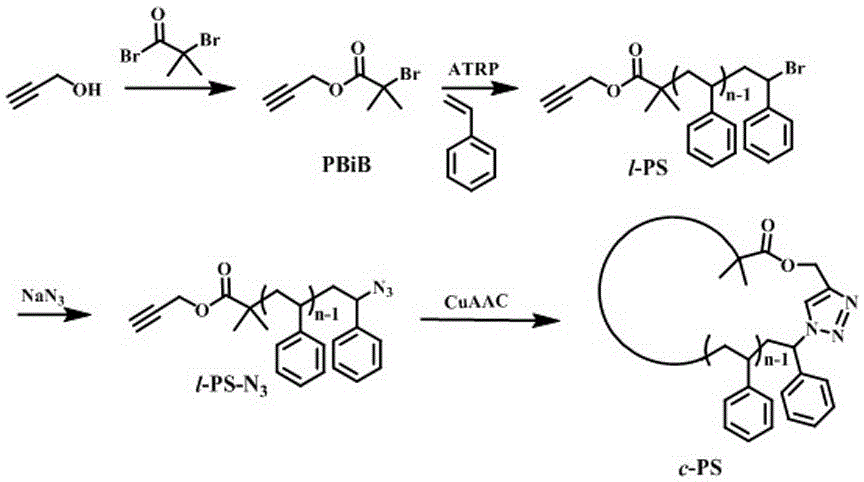
[0077] Embodiment 1: Cyclic polystyrene ( c -PS) synthesis (specific synthetic route such as figure 1 shown).
[0078] Add 5.6g (0.1mol) of propynyl alcohol and 100mL of dichloromethane solvent into a 250mL three-necked flask, then add 12.2g (0.12mol) of triethylamine, cool it down to 0~5°C with an ice-water bath, and then Slowly add 27.6g (0.12mol) 2-bromoisobutyryl bromide into the reaction bottle, stir for 30min after the dropwise addition, remove the ice-water bath and react for 12h, filter with suction, wash the filtrate with water, saturated sodium carbonate solution, and saturated saline Once, dried with anhydrous sodium sulfate for 2 h, concentrated to obtain a crude product, and then distilled under reduced pressure to obtain 10.2 g of the final product PBiB, yield: 50%.
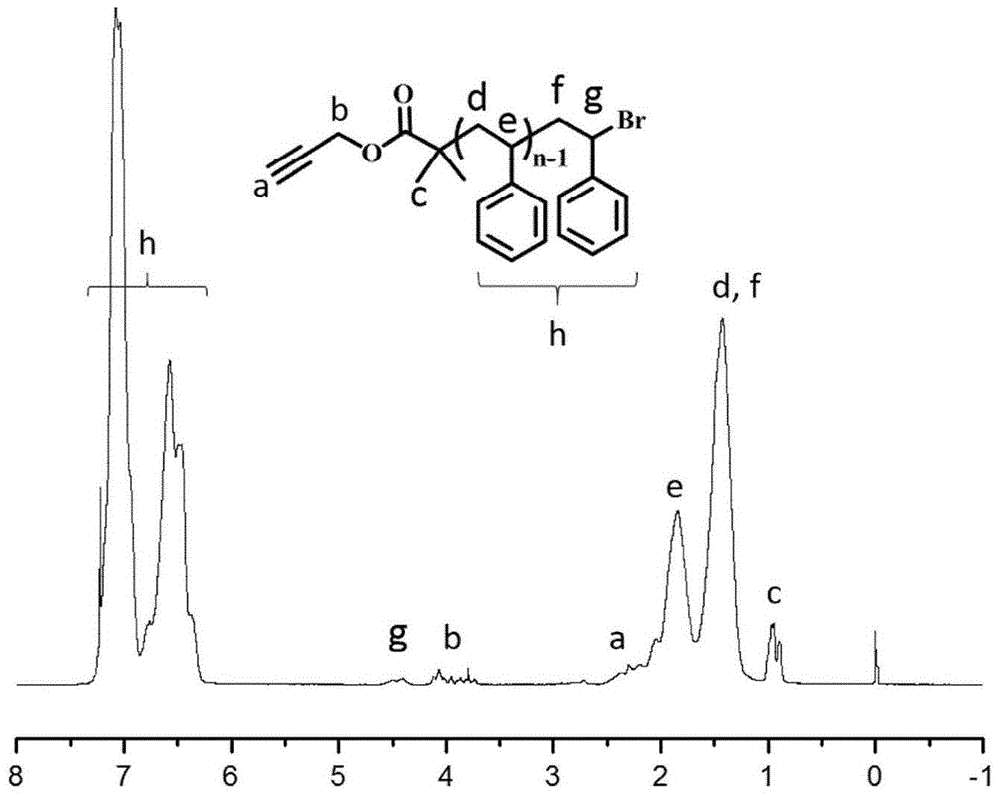
[0079] 9.06g (87.0mmol) St, 178.4mg (0.87mmol) PBiB, 55.8mg (0.25mmol) CuBr 2 , 130.0mg (0.75mmol) PMDETA, 87.5mg (0.5mmol) VC and 10mL anisole were added to a 25mL Schlenk bottle, and reacted i...
Embodiment 2
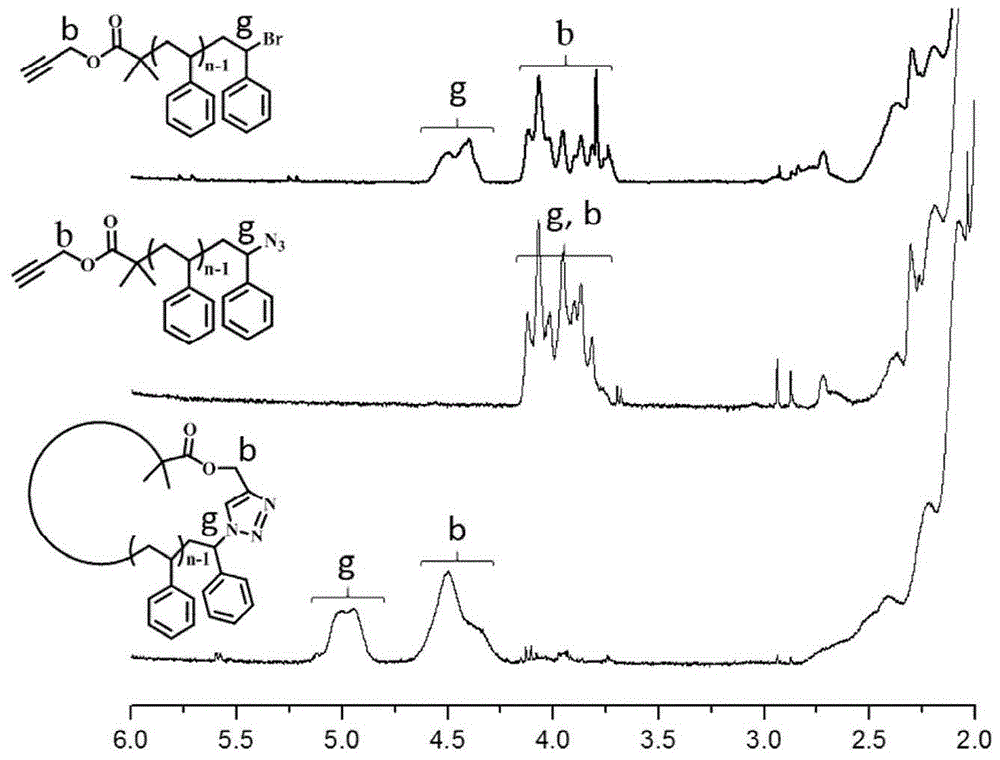
[0082] Embodiment 2: the bromination of cyclic polystyrene (reaction scheme is referring to Figure 5 ).
[0083] 300mg (repeating unit 2.88mmol) c -PS, 153.8mg (0.86mmol) NBS, 94.58mg (0.58mmol) AIBN and 50mL CCl 4 Add it into a 100mL round-bottomed flask, stir at room temperature for 30min, and heat to 80°C under reflux to react overnight. Suction filter the reaction solution to remove the floating flocs in the upper layer, wash the filtrate with 0.1M sodium thiosulfate solution and water several times, concentrate the organic phase, precipitate it with methanol and filter it with suction, dissolve it with THF, precipitate it with methanol and filter it with suction. Vacuum drying for 24h gave 238.0 mg of yellow powdered brominated cyclic polystyrene ( c -PS-Br). Use NBS / AIBN / CCl in this process 4 system, the bromine content in the polymer can be adjusted by adjusting the ratio of styrene repeating unit to NBS and AIBN, so as to control the number of side chains of th...
Embodiment 3
[0085] Embodiment 3: Brominated cyclic polystyrene initiates St polymerization as a macroinitiator (reaction scheme sees Figure 5 ).
[0086]10.31mg (0.087mmol) brominated cyclic polystyrene macromolecular initiator, 12.45mg (0.087mmol) cuprous bromide, 0.9060g (8.7mmol) styrene monomer, 15.08 (0.087mmol) PMDETA ligand Add it into a 5mL ampoule bottle, use anisole as the solvent, and seal the tube under an oxygen-free atmosphere after pumping out the gas several times at low temperature. Place the sealed ampoule in a heating mantle at 90°C, and react according to the predetermined time (1-8 days). Immediately after the reaction, cool the sealed tube with cold water, open the sealed tube and dissolve the contents with tetrahydrofuran, pour it into 250mL of methanol and place it overnight, filter it with suction, and dry it to obtain ring brush-shaped polystyrene with different side chain lengths. This process requires strict oxygen removal. During the polymerization process,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com