A kind of preparation method of cyclic macromolecular chain transfer agent and ring comb polymer
A technology of macromolecular chain and transfer agent, which is applied in the field of polymer material synthesis, can solve the problems of difficult to adjust the graft density of cyclic comb polymers, and no cyclic comb polymers are seen, so as to achieve a rich variety of polymers. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of embodiment one cyclic macromolecular chain transfer agent
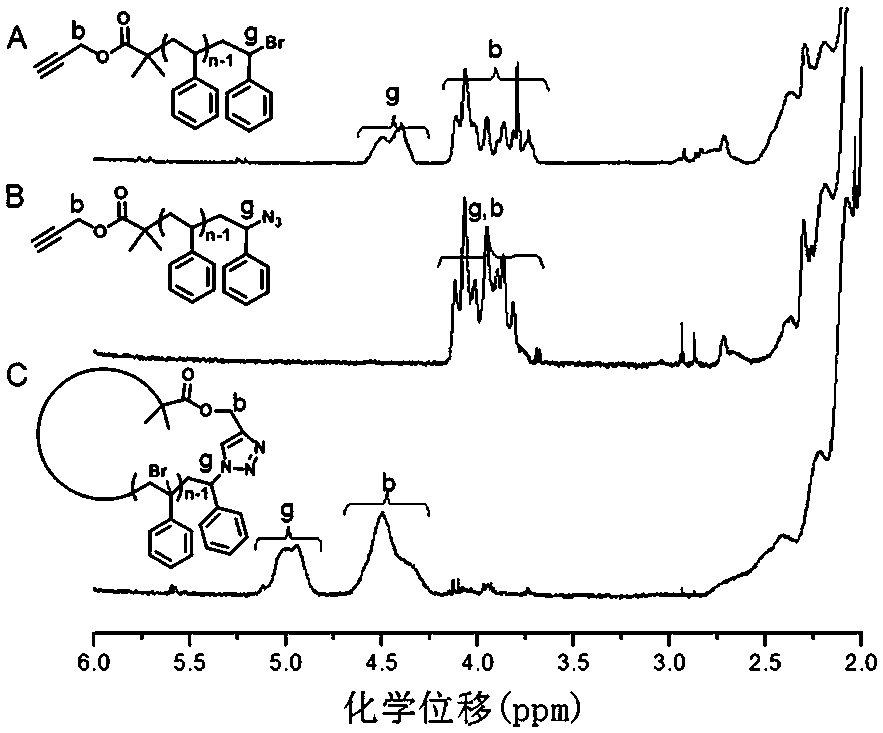
[0049] Add 5.6g (0.1mol) of propynyl alcohol and 100mL of dichloromethane solvent into a 250mL three-necked flask, then add 12.2g (0.12mol) of triethylamine, cool it down to 0~5°C with an ice-water bath, and then Slowly add 27.6g (0.12mol) 2-bromoisobutyryl bromide into the reaction bottle, stir for 30min after the dropwise addition, remove the ice-water bath and react for 12h, filter with suction, wash the filtrate with water, saturated sodium carbonate solution, and saturated saline Once, dried with anhydrous sodium sulfate for 2 h, concentrated to obtain a crude product, and then distilled under reduced pressure to obtain 10.2 g of the final product PBiB, yield: 50%.
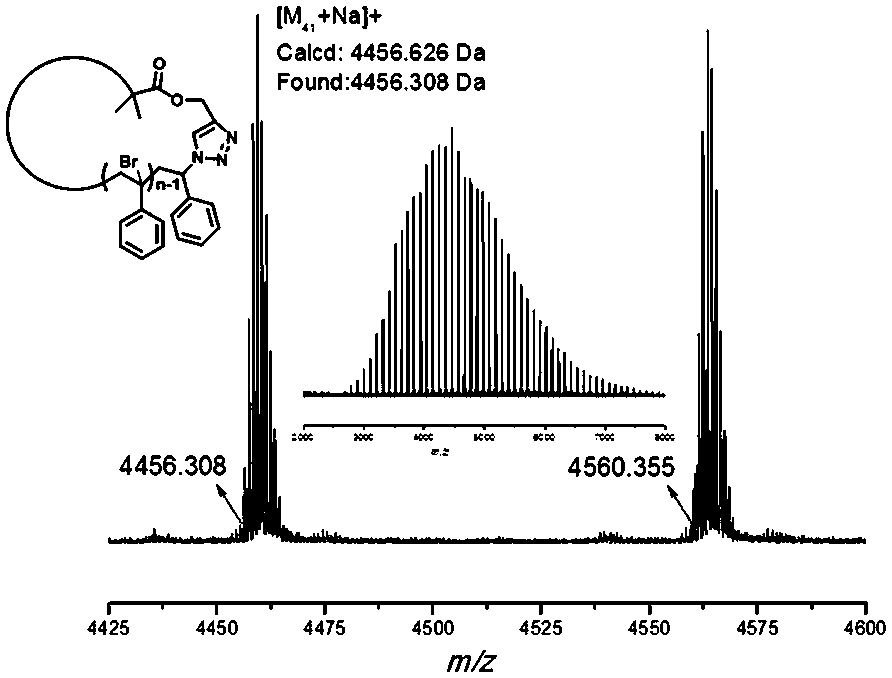
[0050] To 25 mL of In a Schlenk bottle, react in an oil bath at 90°C for 5h. After the reactant was dissolved in THF, the copper salt was removed through a neutral alumina column, then precipitated with methanol and suction filt...
Embodiment 2
[0060] Add St, 2-propargyl bromopropionate, copper iodide, 2,2'-bipyridine, phenol and 10 mL anisole into a 25 mL Schlenk bottle, and react in an oil bath at 110 °C for 5 h. After the reactant was dissolved in THF, the copper salt was removed through a neutral alumina column, then precipitated with methanol and suction filtered, and dried in a vacuum oven for 24 hours to finally obtain a white powdered linear polystyrene ( l -PS); will l -PS, NaN 3 Add DMF and DMF into a 25mL round-bottom flask, react in an oil bath at 25°C for 24h, dissolve the reactant in THF, remove the azide reagent through a neutral alumina column, precipitate with methanol and suction filter, vacuum box After drying for 24 hours, the white powdery azide linear polystyrene ( l -PS-N 3 ); Add toluene into a 1000mL three-necked flask, pass through nitrogen to remove oxygen overnight, add triethylamine, tripropylene glycol methyl ether acetate and cuprous chloride to the 1000mL three-necked flask in seque...
Embodiment 3
[0061] The preparation of embodiment tricyclic comb polymer
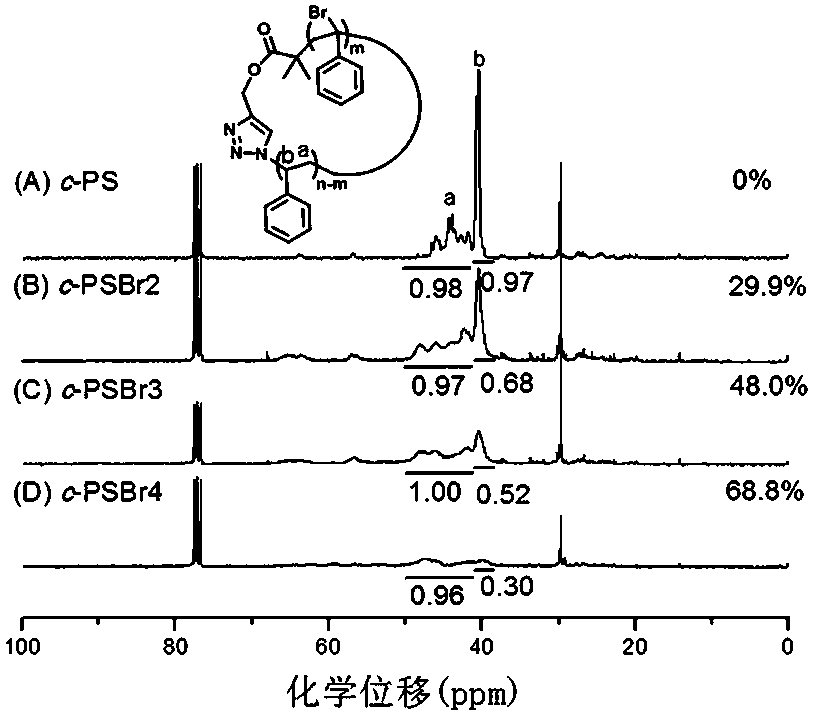
[0062] in turn c -PSN(8.39mg, 0.048mmol), St(1.00g, 9.6mmol), AIBN(3.94mg, 0.024mmol) and toluene (1mL) were added to a 5mL ampoule, and after several times of pumping at low temperature, Seal the tube under an oxygen-free atmosphere. Place the sealed ampoule in a heating mantle at 90°C, and react according to the predetermined time (1-48 hours). Immediately after the reaction, cool the sealed tube with cold water. After opening the sealed tube, dissolve the polymer with tetrahydrofuran, pour it into 250 mL of methanol and leave it overnight, filter it with suction, and dry it to obtain ring-comb polystyrene with different side chain lengths. c -PSN- g -PS. This process requires strict oxygen removal. During the polymerization process, the molecular weight of the polymer can be controlled by adjusting the reaction time. For details, see Figure 5 and Table 2.
[0063] Table 2 c - Polymerization results of PS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com