Incomplete antibody screening colloidal gold reagent kit and preparation method thereof
A complete antibody and colloidal gold technology, used in biological testing, material testing products, measuring devices, etc., can solve the problems of reduced red blood cell antigen activity, long detection time (more than 60 minutes, no more than 3 months, etc.) Scale operation, clear and easy to judge results, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
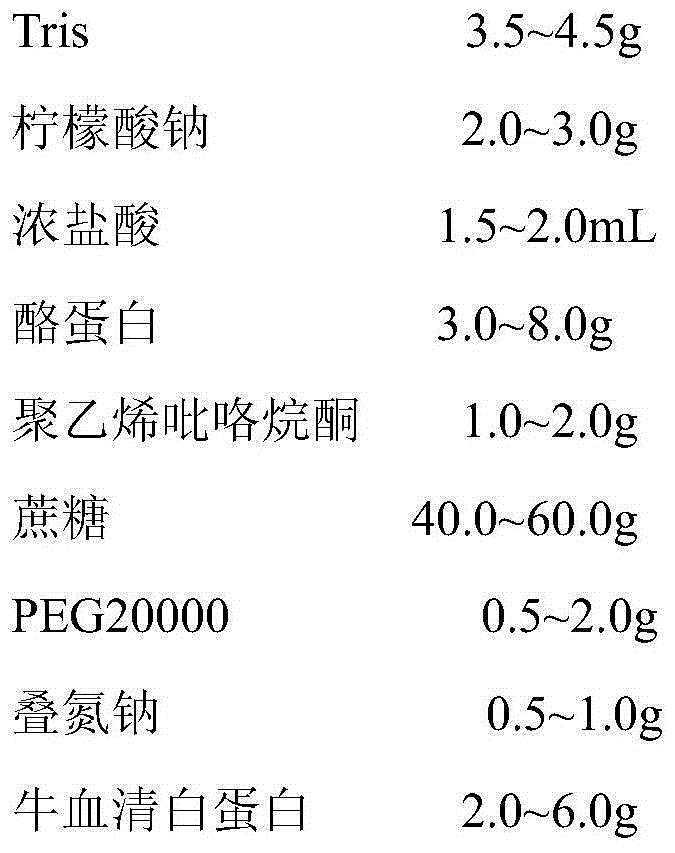
[0042] A colloidal gold kit for erythrocyte antibody screening, the preparation method of the kit comprises the following steps:
[0043] (1) chloroauric acid is polymerized into colloidal gold particles with a size of 40 to 60 nm under the action of a reducing agent, and the reducing agent is at least one of sodium citrate, white phosphorus, ascorbic acid and tannic acid;
[0044] (2) Use monoclonal IgM antibody or polyclonal antibody to detect and identify healthy adult O red blood cells that are negative in the direct anti-human globulin test, and obtain common coverage of D, C, E, c, e, Jk a 、JK b , M, N, S, s, Fy a 、Fy b 、Di a , K, k, Le a 、Le b The first red blood cell, the second red blood cell and the third red blood cell of the P1 antigen, specifically the antigen spectrum of the first red blood cell is D, C, E, e, Jk a 、JK b , M, N, S, Fy a 、Di a , k, Le b and P1 antigen, the antigen spectrum of the second red blood cell is D, E, c, Jk a , M, s, Fy a , K,...
Embodiment 2
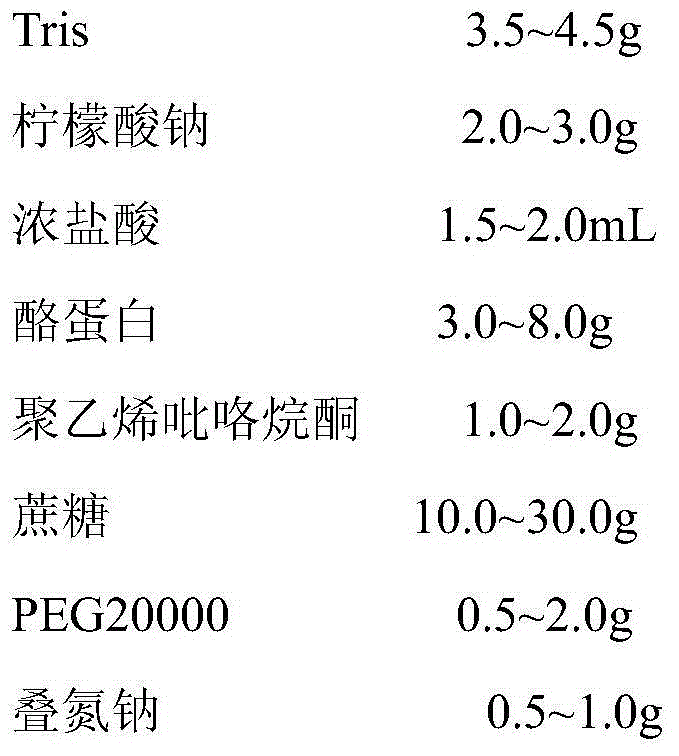
[0067] A colloidal gold kit for erythrocyte antibody screening, the kit is a dry kit, and its preparation method comprises the following steps:
[0068] (1) chloroauric acid is polymerized into colloidal gold particles with a size of 40 to 60 nm under the action of a reducing agent, and the reducing agent is at least one of sodium citrate, white phosphorus, ascorbic acid and tannic acid;
[0069] (2) Through the detection and identification of healthy adult O red blood cells negative in the direct anti-human globulin test by monoclonal IgM antibody or polyclonal antibody, the common coverage of D, C, E, c, e, Jk is obtained a 、JK b , M, N, S, s, Fy a 、Fy b 、Di a , K, k, Le a 、Le b The first red blood cell, the second red blood cell and the third red blood cell of the P1 antigen, specifically the antigen spectrum of the first red blood cell is D, C, E, e, Jk a 、JK b , M, N, S, Fy a 、Di a , k, Le b and P1 antigen, the antigen spectrum of the second red blood cell is D,...
Embodiment 3
[0095] Sensitivity test result of colloidal gold kit for screening red blood cell antibody of the present invention
[0096] Use the registered blood group monoclonal antibody (IgG) reagent to react with the colloidal gold particles containing the corresponding antigen in this kit (not limited to these antigens), and reach the following standards:
[0097] antiserum
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com