Polyimide, and alicyclic tetracarboxylic acid dianhydride for use in production of same
A technology of tetracarboxylic dianhydride and polyimide, applied in the direction of organic chemistry, etc., can solve problems such as difficulty, difficulty in practical application, and polyimide polymerizability is not necessarily sufficient, and achieve light transmittance or heat resistance. The effect of excellent performance and low coefficient of linear expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0230] Hereinafter, although this invention is demonstrated more concretely based on an Example and a comparative example, this invention is not limited to a following example.
[0231] First, methods for evaluating properties of compounds, thin films, and the like obtained in each synthesis example, each example, and each comparative example will be described.
[0232]
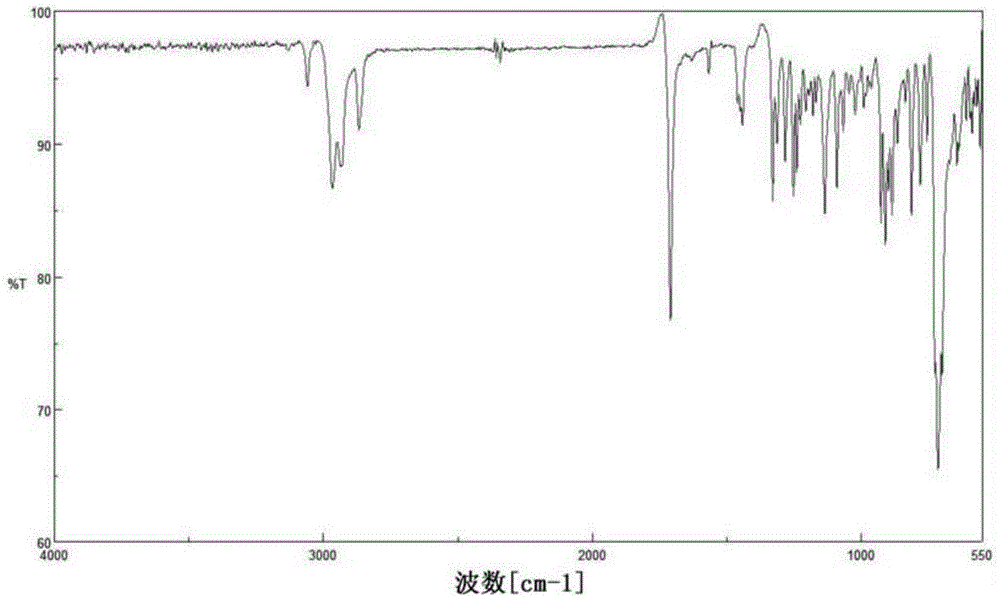
[0233] The identification of the molecular structure of the compound obtained in each synthesis example and each example etc. is by using an infrared spectroscopic analyzer (manufactured by JASCO Corporation, FT / IR-460, FT / IR-4100, manufactured by Thermo Fisher Scientific K.K., NICOLET 380FT-IR) and an NMR measuring machine (manufactured by VARIAN, trade name: UNITY INOVA-600 and JNM-Lambda 500 manufactured by JEOL Ltd.) to measure IR and NMR spectra.
[0234]
[0235] The identification of the isomer type of the compound obtained in each synthesis example and each Example etc. was performed by HPLC measu...
Synthetic example 1
[0248] (Synthesis Example 1: Preparation of 5-norbornene-2-spiro-α-cyclopentanone-α’-spiro-2”-5”-norbornene)
[0249] First, 61.7g (0.757mol) of dimethylamine hydrochloride, 182g (2.46mol) of 1,3-dioxolane, and 25.9g (0.308mol) of cyclopentanone were added to a 2L three-necked flask , 4.0 g (38 mmol) of 35% hydrochloric acid. Next, after attaching a spherical condenser to the three-necked flask, the internal atmosphere of the three-necked flask was replaced with nitrogen. Thereafter, the three-necked flask was submerged in an oil bath at 90° C., heated and stirred for 5 hours to obtain a mixture containing the Mannich base (represented by the general formula (I-2) described in the above reaction formula (I). The compound [n in general formula (I-2) is 2, R 2 and R 3 are hydrogen, R are methyl, and X - For the compound of chloride ion]) reaction solution. In addition, gas chromatographic analysis (GC analysis: using Agilent Technologies Inc. trade name "6890N" as a detecto...
Synthetic example 2
[0254] (Synthesis Example 2: Preparation of norbornane-2-spiro-α-cyclopentanone-α’-spiro-2”-norbornane-5,5”,6,6”-tetracarboxylic acid tetramethyl ester)
[0255] Add methanol (600ml) and 61.1g (454mmol) of CuCl 2 (II), 26.0 g (108 mmol) of 5-norbornene-2-spiro-α-cyclopentanone-α'-spiro-2”-5”-norbornene obtained in Synthesis Example 1 and 243 mg (1.08 mmol) of Pd(OAc) 2 After obtaining the mixed solution, the container was sealed, and the atmosphere inside was replaced with nitrogen. Next, while introducing carbon monoxide into the container, the mixed liquid was stirred at 20° C. and 0.9 MPa for 5 hours to obtain a reaction liquid. Next, carbon monoxide was removed from the inside of the container, and the reaction liquid was concentrated with an evaporator, whereby methanol was removed from the reaction liquid to obtain a reaction product. Thereafter, toluene (900 ml) and 5% by mass hydrochloric acid (900 ml) were added to the reaction product, and vigorously stirred at 80...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| visible light transmittance | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com