Typhoid and paratyphoid A and B polysaccharide-protein conjugated polyvalent combined vaccine
A polysaccharide protein, type A and type B technology, applied in the direction of carrier binding antigen/hapten components, drug combinations, bacterial antigen components, etc., can solve the problem that vaccines cannot provide population protection, reduce the number of times, simplify immunization procedures, protect Wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
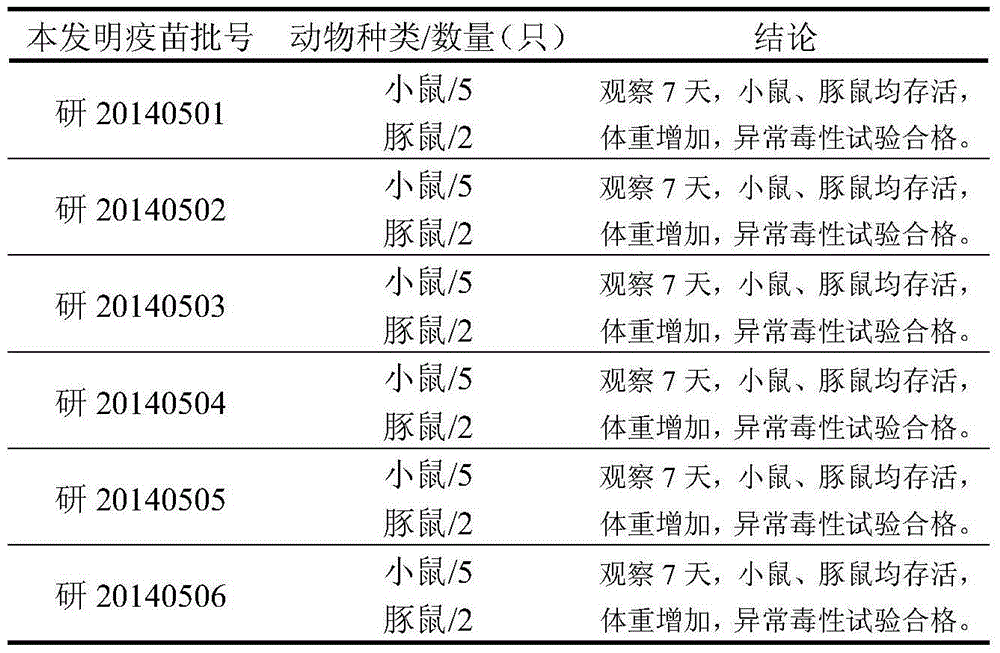
[0022] Preparation of Example 1 Typhoid Vi polysaccharide, Paratyphoid A O-SP and Paratyphoid B O-SP
[0023] 1.1 Preparation of typhoid Vi polysaccharide
[0024] Salmonella typhi was cultured by fermentation in large tanks. After formaldehyde was sterilized, the culture supernatant of Salmonella typhi culture was harvested by centrifugation, and typhoid fever was initially extracted from the supernatant of Salmonella typhi culture by cetyl ammonium bromide method. Vi polysaccharide, and then through cold phenol extraction or column chromatography, the typhoid Vi polysaccharide is refined and purified. The cetyl ammonium bromide method and cold phenol extraction were according to the methods in the "Chinese Pharmacopoeia" 2010 edition three monographs in the production and inspection regulations of typhoid Vi polysaccharide vaccine.
[0025] 1.2 Preparation of paratyphoid A O-specific polysaccharide (ie paratyphoid A O-SP), type B paratyphoid fever O-specific polysaccharide ...
Embodiment 2
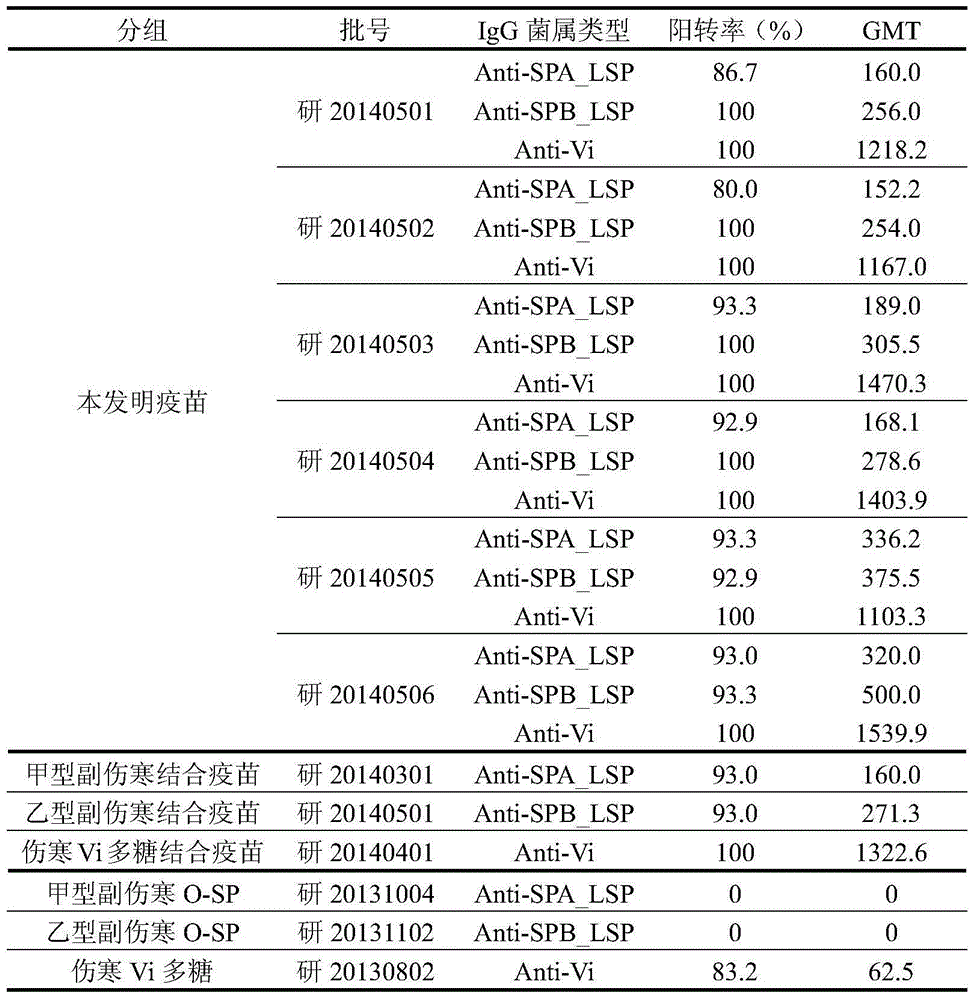
[0030] Example 2 Preparation of typhoid Vi polysaccharide protein conjugate stock solution, paratyphoid fever O-SP protein conjugate stock solution and paratyphoid fever B O-SP protein conjugate stock solution
[0031] 1. Preparation of paratyphoid A O-SP-TT conjugate stock solution and paratyphoid B O-SP-TT conjugate stock solution
[0032]O-SP reacts with adipic acid dihydrazide (ADH) to form O-SP-ADH derivatives, tetanus toxoid (TT) is purified by column chromatography, and the purified TT is mixed with O-SP-ADH to obtain a total Conjugation reaction to obtain O-SP-ADH-TT conjugates. The conjugate is purified by column chromatography to remove impurities to obtain the stock solution of the O-SP-TT conjugate. The raw solutions of paratyphoid O-SP-TT conjugates were purified by column chromatography, sterilized by 0.2 μm membrane filtration and preserved. The preparation method of each paratyphoid O-SP-TT conjugate stock solution is the Synthesis, Characterization, and Immu...
Embodiment 3
[0035] Embodiment 3 Vaccine finished product preparation
[0036] 1. Preparation of auxiliary materials
[0037] ① 0.1mol / L sodium hydroxide: Weigh 1 gram of sodium hydroxide in a 500ml container, dry-roast to depyrogenate, add appropriate amount of water for injection to dissolve, cool to room temperature and set the volume to 250ml.
[0038] ②Preparation of glycine solution: Weigh 70 grams of pharmaceutical grade glycine, dissolve it in an appropriate amount of water for injection, adjust the pH to 7.3±0.1 with 0.1mol / L sodium hydroxide, adjust the volume to 350ml, and sterilize and filter with a 0.2μm membrane. have to.
[0039] ③Sodium chloride solution: Weigh 5 grams of pharmaceutical grade sodium chloride into a 500ml container, dry-roast to depyrogenate, add an appropriate amount of water for injection to dissolve, and set the volume to 250ml.
[0040] ④0.01mol / L Phosphate Buffer (PB Buffer): Weigh 0.276 grams of disodium hydrogen phosphate (heptahydrate), 0.063 grams...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com