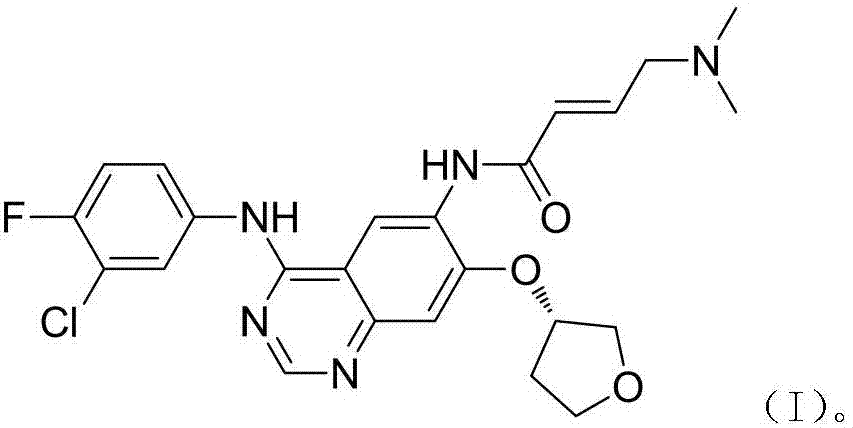
A kind of preparation method of Afatinib intermediate
A fluorophenyl and amino technology, applied in the field of pharmaceutical synthesis, can solve the problems of difficult removal of residues, low purity, environmental pollution, etc., and achieve the effects of low price and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Take 1.0g 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 1.66g potassium hydroxide , 2.22g of D-glucose, 10mL of dimethyl sulfoxide, and 10mL of water were added to the reaction flask, and heated to 100°C. After the reaction was complete, 30 mL of water was added dropwise, then the temperature was lowered to 0° C., and suction filtered after 1 hour, the filter cake was washed with 20 mL of water. get N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine 0.83 g, purity 97.62%.
Embodiment 2
[0019] Take 1.0g 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 1.66g potassium hydroxide , 2.18g of vitamin C, 10mL of dimethyl sulfoxide, and 10mL of water were added to the reaction flask, and heated to 100°C. After the reaction was complete, 30 mL of water was added dropwise, then the temperature was lowered to 0° C., and suction filtered after 1 hour, the filter cake was washed with 20 mL of water. get N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine 0.79 g, purity 99.24%.
Embodiment 3
[0021] Take 0.5g 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, 0.84g potassium hydroxide , 1.09g of vitamin C, 10mL of N,N-dimethylacetamide, and 10mL of water were added to the reaction flask, and heated to 100°C. After the reaction was complete, N 4 -(3-Chloro-4-fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazolinediamine 0.4 g, purity 98.31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com