Rosin derivate for soldering flux and preparation method
A technology for rosin derivatives and flux, which is applied in the field of rosin derivatives for flux and its preparation, can solve the problems of poor solvent solubility, high preparation cost, poor thermal stability, etc. good film effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The first step, reduction: Take 10g of dehydroabietic acid, dissolve it in 20g of anhydrous tetrahydrofuran, slowly add 2.7g of lithium aluminum hydride at 0°C, return to room temperature after the addition, and heat to reflux for 1h, then cool to 0°C , slowly drop 50g of 20% H under stirring 2 SO 4 The solution was extracted 3 times with ethyl acetate, the ethyl acetate layer was collected, and the solvent was recovered by distillation under reduced pressure to obtain dehydroabietyl alcohol with a yield of 97% and a GC content of 97%;
[0032] The second step, esterification: take 10g of dehydroabietyl alcohol, dissolve it in 15g of tetrahydrofuran, add 3.9g of succinic anhydride, 0.01g of anhydrous sodium acetate in turn, stir and reflux for 4 hours, and recover the solvent by distillation under reduced pressure to obtain dehydroabietyl alcohol. Hydroabietyl Succinate Monoester.
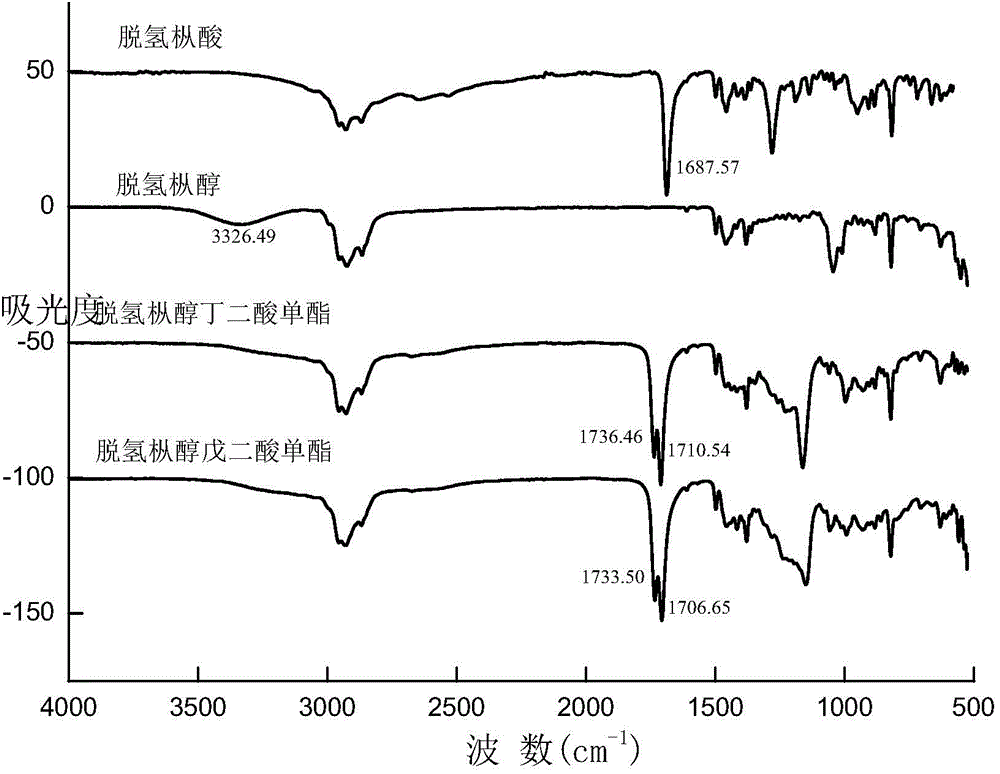
[0033] Depend on figure 1 It can be seen from the infrared characteristic absorption ...
Embodiment 2
[0037] The first step, reduction: Take 10g of dehydroabietic acid, dissolve it in 30g of anhydrous tetrahydrofuran, slowly add 3g of lithium aluminum hydride at 2°C, return to room temperature after the addition, and heat to reflux for 0.8h, then cool to 2°C , slowly dropwise added 60g of 15% H under stirring 2 SO 4 The solution was extracted 3 times with ethyl acetate, the ethyl acetate layer was collected, and the solvent was recovered by distillation under reduced pressure to obtain dehydroabietyl alcohol with a yield of 96% and a GC content of 98%;
[0038] The second step, esterification: take 10g of dehydroabietyl alcohol, dissolve it in 17g of tetrahydrofuran, add 4g of succinic anhydride, 0.02g of anhydrous sodium acetate in turn, reflux under stirring for 5 hours, and recover the solvent by distillation under reduced pressure to obtain dehydrogenation Abityl Succinate Monoester. Gained spectrum feature is identical with embodiment 1 spectrum, in order to avoid dupli...
Embodiment 3
[0040] The first step, reduction: Take 10g of dehydroabietic acid, dissolve it in 40g of anhydrous tetrahydrofuran, slowly add 3.8g of lithium aluminum hydride at -1°C, return to room temperature after the addition, and heat to reflux for 1.2h, then cool to -1°C, slowly drop 65g of 12% H under stirring 2 SO 4 The solution was extracted 3 times with ethyl acetate, the ethyl acetate layer was collected, and the solvent was recovered by distillation under reduced pressure to obtain dehydroabietyl alcohol with a yield of 98% and a GC content of 97%;
[0041] The second step, esterification: take 10g of dehydroabietyl alcohol, dissolve it in 20g of tetrahydrofuran, add 4.2g of succinic anhydride, 0.03g of anhydrous sodium acetate in turn, reflux under stirring for 6 hours, and recover the solvent by distillation under reduced pressure to obtain dehydroabietyl alcohol. Hydroabietyl Succinate Monoester. Gained spectrum feature is identical with embodiment 1 spectrum, in order to av...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com