High-stability recombinant procalcitonin and preparation method and application thereof
A procalcitonin, high stability technology, applied in the direction of calcitonin, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of easy degradation, instability, affecting the accuracy of test results, etc. Efficient expression and the effect of maintaining spatial conformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
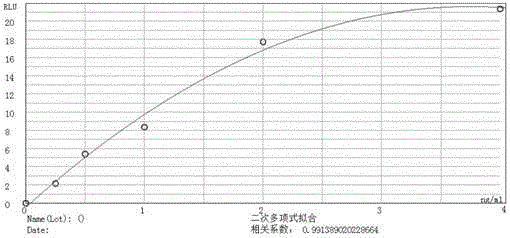
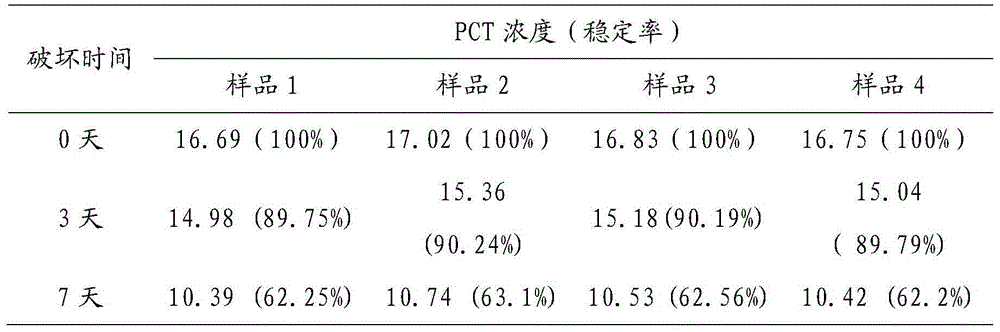
Image
Examples
Embodiment 1
[0034] Example 1 Design of High Stability PCT Antigen Amino Acid Sequence
[0035] Download the natural PCT amino acid sequence GI:76880474 from the NCBI website, and replace PCT at the 58-59aa hydrolysis site (-Lys -Arg-) and the hydrolysis site (-Gly-Lys-Lys-Arg-) at 92-95aa to obtain the amino acid sequence of PCT with high stability (such as 1 in the sequence listing). The PCT amino acid sequence after replacement is compared with the original sequence as figure 1 Shown, where different amino acids are shown with "*" and identical amino acids with "=".
Embodiment 2
[0036] Example 2 Codon optimization and acquisition of high stability PCT antigen gene
[0037] Sequence 1 was reverse-translated into a nucleotide sequence using dominant codons of Escherichia coli, namely the gene sequence of high-stability PCT (such as 2 in the sequence listing).
[0038] Considering the correct rate of current domestic gene primer synthesis, the present invention designs PCT gene into two sections A and B, and 6 primers are designed for each section of gene to be synthesized respectively, each primer end has a matching sequence of 16 nucleotides, each The sequences of primers synthesized by paragraph 3-14 are shown in the sequence listing. In order to describe the preparation method conveniently, the corresponding primer sequences are named, and SEQ ID NO:3 to 8 are respectively named as AF1, AR1, AF2, AR2, AF3, AR3; SEQ ID NO:9 to 14 are respectively named as BF1, BR1, BF2, BR2, BF3, BR3;
[0039] The method is as follows:
[0040] The synthesis of the...
Embodiment 3
[0041] Example 3 Cloning, expression and labeling of highly stable PCT antigen gene
[0042] 1. Construction of highly stable PCT antigen expression plasmid
[0043] 1.1 PCR product and expression vector pBVIL-1 plasmid double digestion
[0044] Take 30 μl of the above gene products and pBVIL-1 expression vector and place them in Eeppendorf centrifuge tubes, add 4 μl of 10×buffer (D), 1 μl of XhoI (10u / μl) and XbaI (12u / μl), add sterilized distilled water to 40 μl, and placed in a 37°C water bath for enzyme digestion overnight.
[0045] Agarose gel electrophoresis purification and recovery of enzyme-cleaved products: PCR product and carrier pBVIL-1 are purified with 1.2% agarose gel after double digestion, and the specific method is according to "Molecular Cloning" (Science Press, second version) method. The purified gene is then recovered with a small amount of gel recovery kit produced by Shanghai Huashun Bioengineering Co., Ltd.: cut out the agarose containing the plasmi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com