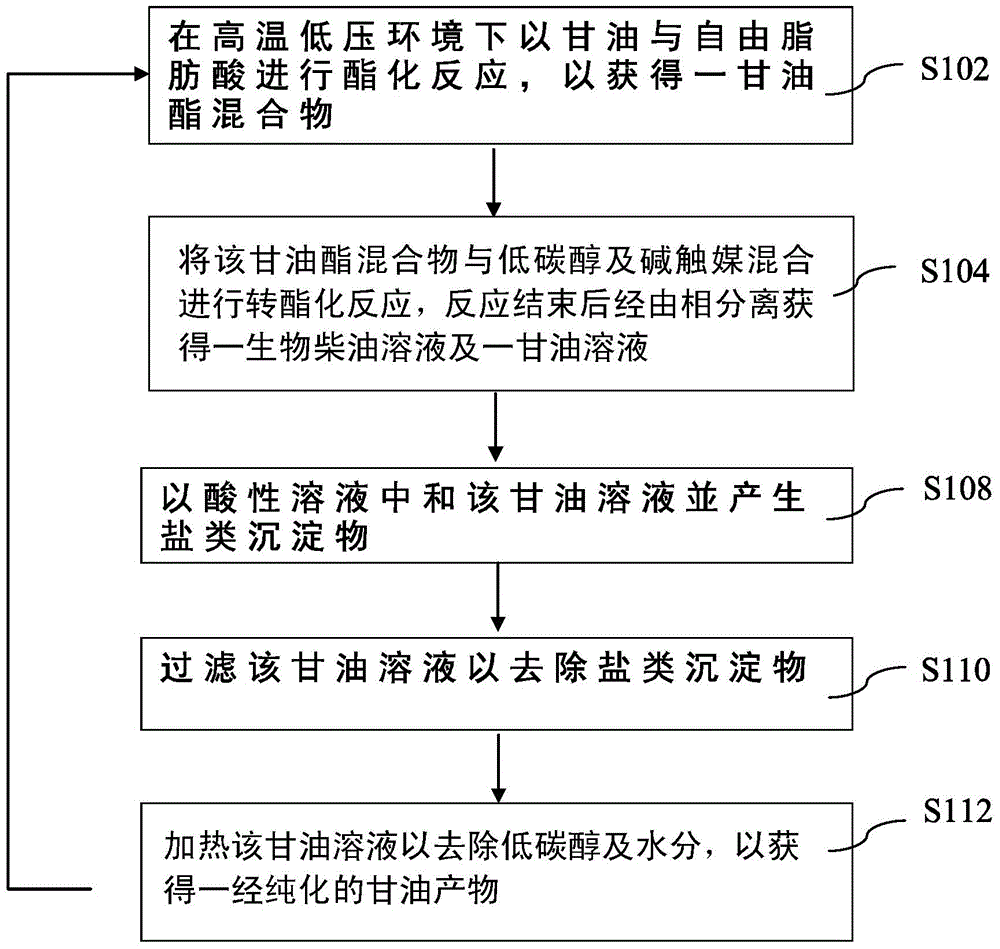
Method for circulating preparation of biodiesel
A biodiesel and cycle preparation technology, applied in the direction of biofuel, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of catalyst loss of activity, high production cost, difficult methanol recovery, etc., to increase added value and save raw materials Cost, improve the effect of corrosion equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
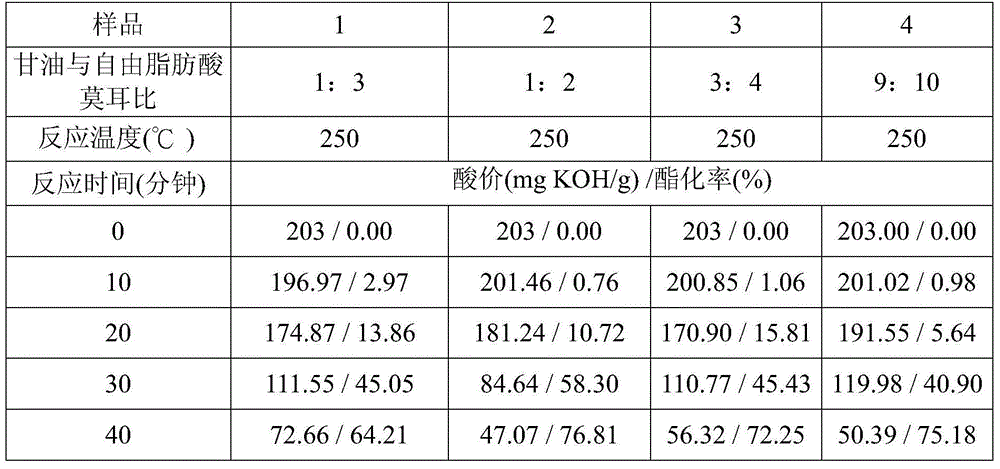
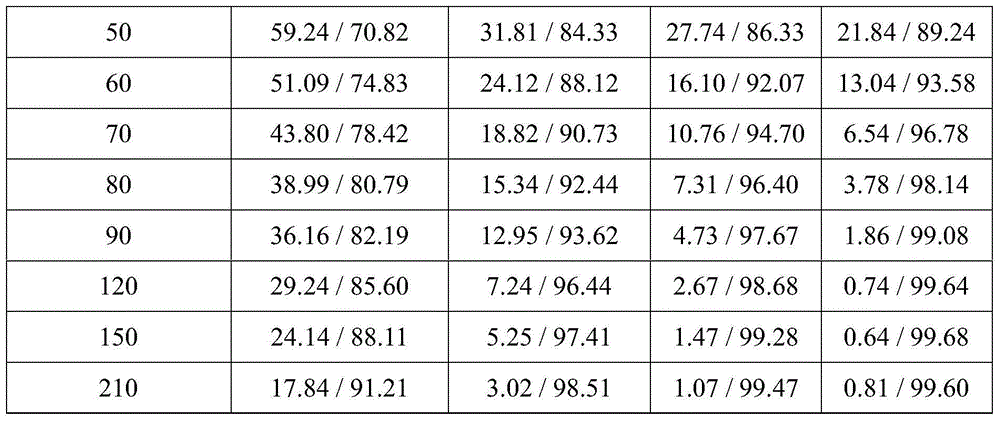
[0026] Example 1 In the high temperature and normal pressure esterification process, the influence of glycerol / free fatty acid molar ratio and reaction temperature on the esterification rate
[0027] In this embodiment, free fatty acid is used as a source of material, and the main fatty acid composition of the free fatty acid is 72wt% oleic acid (oleic acid) and 13wt% linoleic acid (linoleate acid), and glycerin is used as the alcohols of the esterification reaction.
[0028] Glycerin and free fatty acid were esterified at a molar ratio of 1:3 to 2:1 under normal pressure, and the reaction temperature was set at 200°C to 250°C, and the stirring and reaction were carried out at a rotation speed of 200rpm for 210 minutes.
[0029] Table 1 compares the effects of different molar ratios of glycerol and free fatty acids on acid value and esterification rate
[0030]
[0031]
[0032]
[0033] The results are shown in Table 1. Using free fatty acid as the material source, t...
Embodiment 2
[0035] The impact of embodiment 2 low pressure environment on the esterification reaction rate
[0036]In Example 2, the mixture of free fatty acid and palm oil is used as raw material, wherein the palm oil is refined palm oil. This embodiment also uses palm sludge oil and crude palm oil from palm oil factories as raw materials. In addition, this embodiment uses vacuum pumping equipment to maintain the interior of the closed reactor at a negative pressure state, which can make the water generated by the esterification reaction easier to be vaporized into water vapor and removed, thereby improving the efficiency of esterification. The reaction speed, on the other hand, can reduce the partial pressure of oxygen in the reactor to reduce the possibility of oil being oxidized in a high temperature environment.
[0037] Table 2 carries out the data of high temperature and low pressure esterification reaction
[0038]
[0039]
[0040] If sample 3 of Table 1 is compared with ...
Embodiment 3
[0041] In the transesterification process of embodiment 3, compare the impact of different operating conditions on the yield of biodiesel
[0042] The oil product in this example is obtained from the glyceride mixture generated by the esterification reaction of sample 4 in Example 1, and the initial acid value is 0.81 milligrams of potassium hydroxide per gram (mg KOH / g). Generally speaking, if the acid value of the oil is less than 2mg KOH / g, the subsequent transesterification reaction can be carried out directly. In this example, the molar ratio of methanol to glyceride mixture is set between 1:1 and 5:1, and 0.52 (w / w%) to 2w / w% potassium hydroxide is added as a transesterification catalyst, the reaction temperature is between 50° C. and 65° C., and the mixing and reaction are carried out at a rotation speed of 400 rpm for 60 minutes.
[0043] Table 3 compares the impact of different operating conditions on the transesterification reaction
[0044]
[0045] The results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com