Ethylene oligomerization method
An ethylene oligomerization and ethylene technology, applied in the field of olefin polymerization, can solve the problems of unattainability, harsh environmental requirements and high unit price, and achieve the effects of high oligomerization reaction activity, simplified production process and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
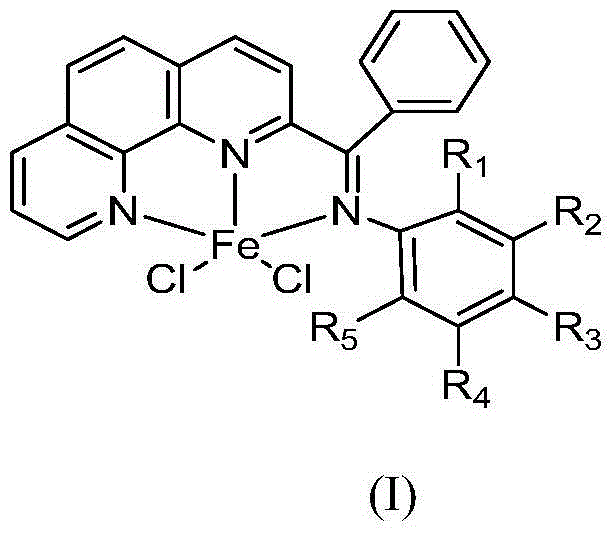
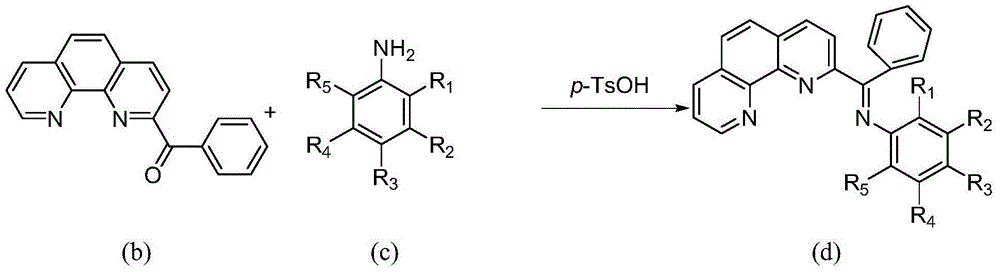
[0039] 1. Catalyst synthesis of 2-benzoyl-1,10-phenanthroline chloride 2,6-diethylanilinate iron (II) complex
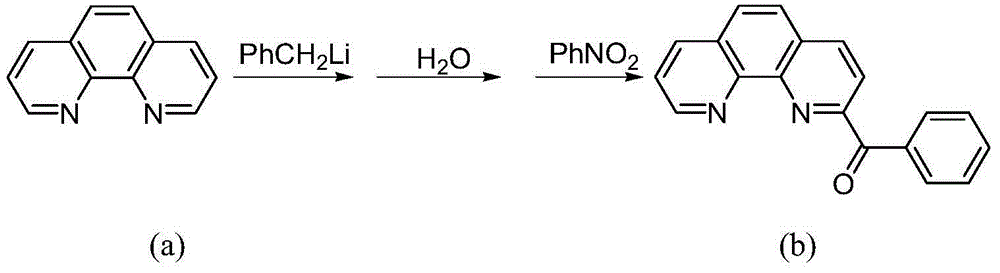
[0040] Synthesis of a.2-benzoyl-1,10-phenanthroline (see the following reaction scheme)
[0041]
[0042]Put 5.1 g (28.3 mmol) of 1,10-phenanthroline into a 250 ml three-necked flask, and dissolve it with 100 ml of toluene under nitrogen protection and magnetic stirring. Slowly add 60ml of benzyllithium 1.2M n-hexane solution (72mmol) dropwise to the three-necked flask under stirring at -60°C, the dropwise addition is completed in about 15 minutes, continue stirring at this temperature for 18h, and then raise the temperature to 30°C About, continue to stir 10h. Then the reaction mixture was cooled to about -30°C, 50ml of distilled water was slowly added thereto, and then heated to 30°C and stirred for 10h. Then separate the liquids, take out the organic phase, and extract the inorganic phase three times with dichloromethane, each time the consumption of dichloro...
Embodiment 2
[0058] The method of Example 1 was adopted to carry out the ethylene oligomerization reaction, the difference being that the weight content of water was 20 ppm based on the weight of the solvent 1-hexene, and the analysis results were shown in Table 1.
Embodiment 3
[0060] The method of Example 1 was adopted to carry out the ethylene oligomerization reaction, the difference being that the weight content of water was 50 ppm based on the weight of the solvent 1-hexene, and the analysis results were shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com