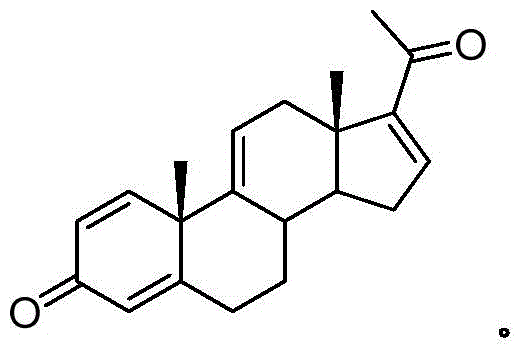
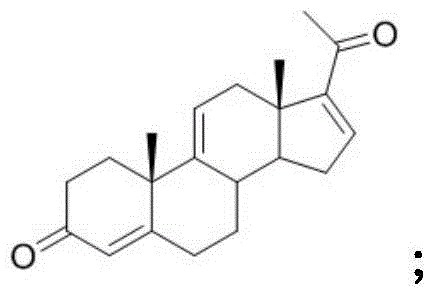
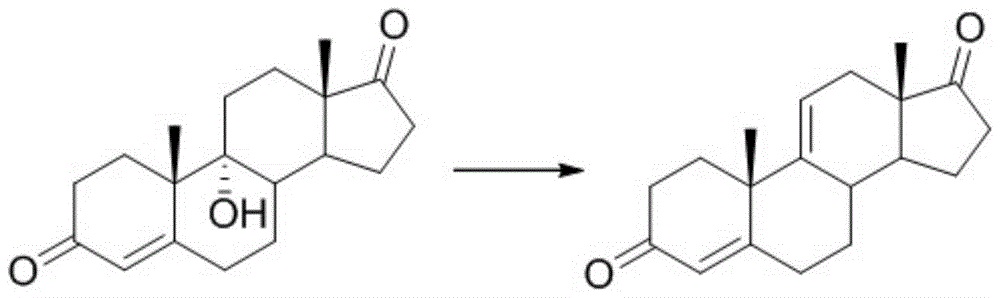
A kind of synthetic method of pregna-1,4,9(11),16(17)-tetraene-3,20-dione and its intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, to achieve the effect of avoiding the use of acetone cyanohydrin, easy to obtain raw materials, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of the first intermediate (androst-4,9(11)-diene-3,17-dione);
[0027]
[0028] At 0°C, trifluoroacetic anhydride (10mL), acetic acid (12mL) and p-toluenesulfonic acid (6.85g) were added to 100mL of dichloromethane, and 9-α-hydroxyandroster (9.0g, 29.8mmol ) in dichloromethane 200mL, stirred at room temperature for 3 hours, added 100mL of water, 5N sodium hydroxide to adjust the pH to about 9, separated the organic layer, extracted the water layer with 100mL×2 dichloromethane, combined the organic layers, After washing with clear water, spin-dried, the first intermediate (7.2g, 85%) was obtained after methanol crystallization, and the nuclear magnetic resonance data of the first intermediate were as follows:
[0029] 1 H NMR (500MHz, CDCl 3 )δ5.76(d,J=1.4Hz,1H),5.63-5.44(m,1H),2.67-2.32(m,6H),2.24-2.03(m,7H),1.73-1.44(m,2H) ,1.35(s,3H),1.17(ddd,J=26.7,12.7,3.9Hz,1H),0.88(s,3H); 13C NMR (125MHz, CDCl3) δ221.18, 199.17, 169.06, 145.16, 124.25...
Embodiment 2
[0030] Example 2: Preparation of the second intermediate (androst-3-methoxy-3,5,9(11)-trien-17-one);
[0031]
[0032] The first intermediate (14 g, 4.9 mmol) was dissolved in tetrahydrofuran (THF) and trimethyl orthoformate ((CH 3 O) 3 CH) mixed solution 150mL, add p-toluenesulfonic acid (1.5g), stir and react at room temperature for 2.5 hours, add water 10mL and triethylamine 3mL, extract with dichloromethane, spin dry, the second intermediate after methanol crystallization body (10.0g, 83%), the nuclear magnetic resonance data of the second intermediate are as follows:
[0033] 1 H NMR (500MHz, CDCl 3 )δ5.52(d, J=5.9Hz, 1H), 5.29(d, J=4.3Hz, 1H), 5.18(s, 1H), 3.58(d, J=5.7Hz, 3H), 2.65-2.45( m, 3H), 2.40(ddd, J=17.7, 13.7, 7.4Hz, 1H), 2.25-2.04(m, 5H), 1.91(ddd, J=25.1, 14.9, 6.7Hz, 2H), 1.67-1.54( m,3H),1.15(s,3H),0.88(s,3H); 13 C NMR (125MHz, CDCl 3 )δ221.90,155.65,144.73,139.00,117.08,115.18,98.25,54.42,49.86,46.36,37.24,36.48,33.42,33.23,32.26,31.76,27.26,25.4...
Embodiment 3
[0034] Example 3: Preparation of the third intermediate (androst-3-methoxy-3,5,9(11)-triene-17-isocyano-p-toluenesulfonylmethylene);
[0035]
[0036] Dissolve p-toluenesulfonylmethylisonitrile (TosMIC) (4.3g, 21.9mmol) in 100mL dry tetrahydrofuran (THF), cool to -50°C, add potassium tert-butoxide tBuOK (2.96g, 26.2mmol), After reacting for 15 minutes, add 30 mL of THF solution of the second intermediate (5.0 g, 16.8 mmol), heat up to -40 to -35 ° C for 2.5 hours, add phosphorous acid (2.15 g, 26 mmol), and react for 15 minutes, add Phosphorus oxychloride (3.6ml, 39mmol), triethylamine (27ml, 194mmol), after heating up to 0°C for 1 hour, pour into 100mL of sodium chloride solution, extract with dichloromethane, spin dry, petroleum ether- Acetone column chromatography obtains the third intermediate (4.95g, 62%), and the nuclear magnetic resonance data of the third intermediate is as follows:
[0037] 1 H NMR (300MHz, CDCl 3 )δ7.83(d, J=8.3Hz, 2H), 7.38(d, J=8.0Hz, 2H), 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com