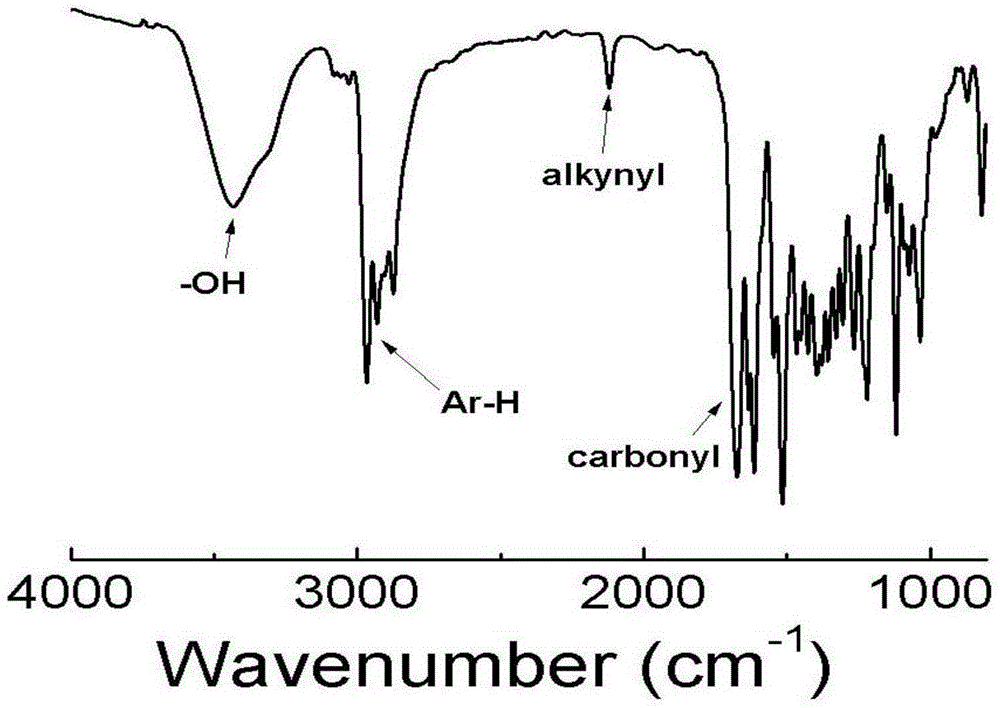
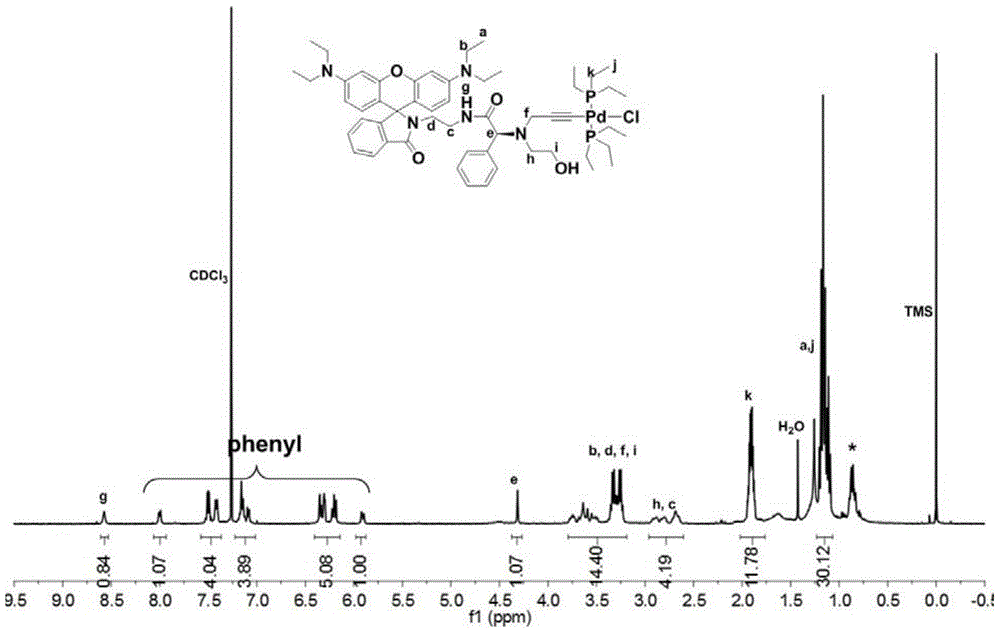
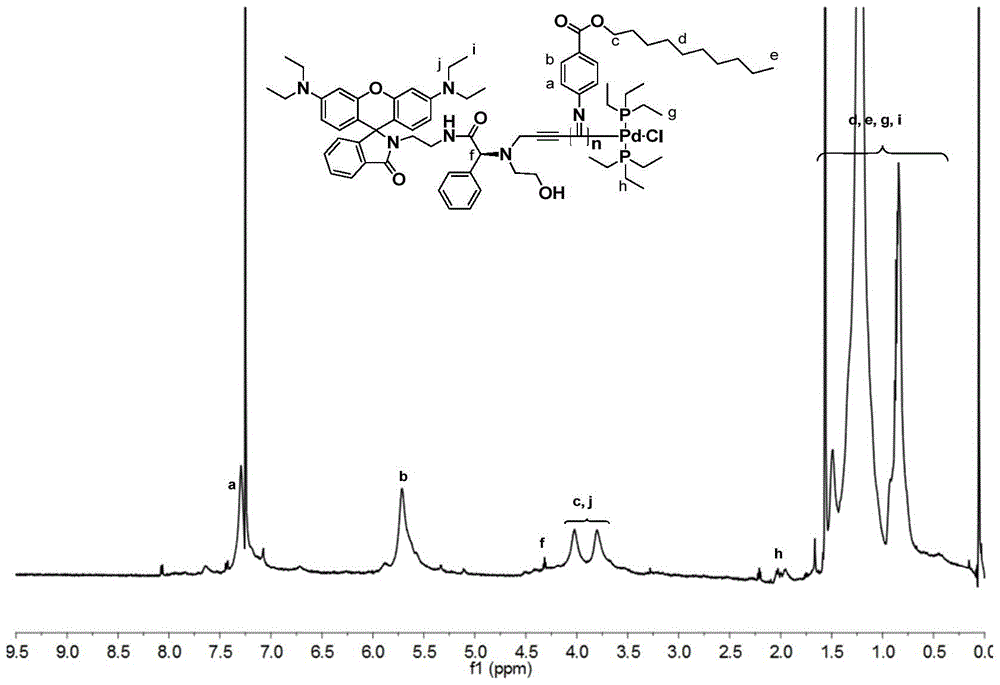
A kind of chiral fluorescent self-classification polymerization bifunctional initiator and its preparation method and application
A self-classification polymerization, bifunctional technology, applied in the field of polymer catalytic synthesis, can solve the problems of difficult implementation of hybrid polymerization, and achieve the effect of narrow molecular weight distribution and uniform molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: the preparation of D-phenylglycine, ethylenediamine initiator
[0079] a. Reflux Rhodamine B and ethylenediamine (excess) in the ethanol solution at 80°C overnight until the fluorescence disappears, spin to dry the ethanol, dissolve the residue with 1M hydrochloric acid and keep stirring, then slowly add 1M NaOH aqueous solution, And continue to stir until a pink precipitate is produced, suction filtered, washed with water, and dried to obtain Intermediate I, the structural formula is as follows:
[0080]
[0081] b. Add D-phenylglycine, di-tert-butyl dicarbonate, and sodium hydroxide to a mixed solvent of tetrahydrofuran and water (2:1) in a molar ratio of 1:1.1:1.2, wherein sodium hydroxide is 10% Stir overnight at room temperature, spin off THF, add dichloromethane, then dropwise add 1M hydrochloric acid solution until the white precipitate in the upper aqueous phase disappears, separate the organic phase, wash twice with water, dry over anhydrous so...
Embodiment 2
[0093] Embodiment 2: Initiate hydrophobic benzene isonitrile polymerization reaction
[0094] The polymerization of benzyl isonitrile is carried out under anhydrous and oxygen-free conditions. In a 10mL polymerization bottle, 1.49 μmol (1.60 mg) of the initiator prepared in Example 1 and 0.10 mmol (28.0 mg) of the benzyl isonitrile monomer are added, and the vacuum and nitrogen gas are repeated. 3 times, add 1.0 mL of dry chloroform, reflux at 60°C for 20 hours, add 10 mL of methanol to quench, and precipitate the polymer, wash 5 times with methanol, centrifuge to obtain a yellow flocculent precipitate, vacuum dry until the quality remains unchanged . Obtain 26.3mg polyisocyanide, its number-average molecular weight is 1.93×10 4 , the molecular weight distribution index is 1.17,
[0095] The structural formula of the benzene isonitrile monomer in the present embodiment is:
[0096]
Embodiment 3
[0097] Embodiment 3: initiate chiral benzene isocyanide polymerization reaction
[0098]The polymerization of chiral benzene isonitrile is carried out under anhydrous and oxygen-free conditions. In a 10mL polymerization bottle, 1.82 μmol (1.96 mg) of the initiator prepared in Example 1 and 0.12 mmol (33.0 mg) of the chiral benzene isonitrile monomer are added. Vacuum nitrogen filling was repeated 3 times, add dry chloroform 1.0mL, reflux reaction at 60°C for 20h, add 10mL methanol to quench, make the polymer precipitate, wash 5 times with methanol, centrifuge to obtain yellow flocculent precipitate, vacuum dry until the quality remains unchanged. Obtain 28.5mg polyisocyanide, its number-average molecular weight is 1.89×10 4 , the molecular weight distribution index is 1.18,
[0099] The structural formula of the benzene isonitrile monomer in the present embodiment is:
[0100]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com