Application of chlorogenic acid in preparation of medicines for treating cardiomyopathy
A kind of cardiomyopathy, chlorogenic acid technology, applied in the field of medicine, to achieve the effect of benefiting recovery, improving shape and inhibiting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1: In vivo pharmacodynamics study on the treatment of dilated cardiomyopathy (DCM) with chlorogenic acid
[0023] 1. Experimental materials
[0024] 1.1 Animals
[0025] There are 110 Wistar rats born two weeks old and breast-fed, half male and half male.
[0026] 1.2 Experimental Drugs and Instruments
[0027] Furazolidone, 10% chloral hydrate, PHLIPsonos7500 color ultrasonic detector, TUNEL apoptosis detection kit, BNP ELISA detection kit, 1 / 10,000 electronic balance, disodium edetate.
[0028] 2. Experimental method
[0029] 2.1 Establishment of rat dilated cardiomyopathy (DCM) model and experimental grouping
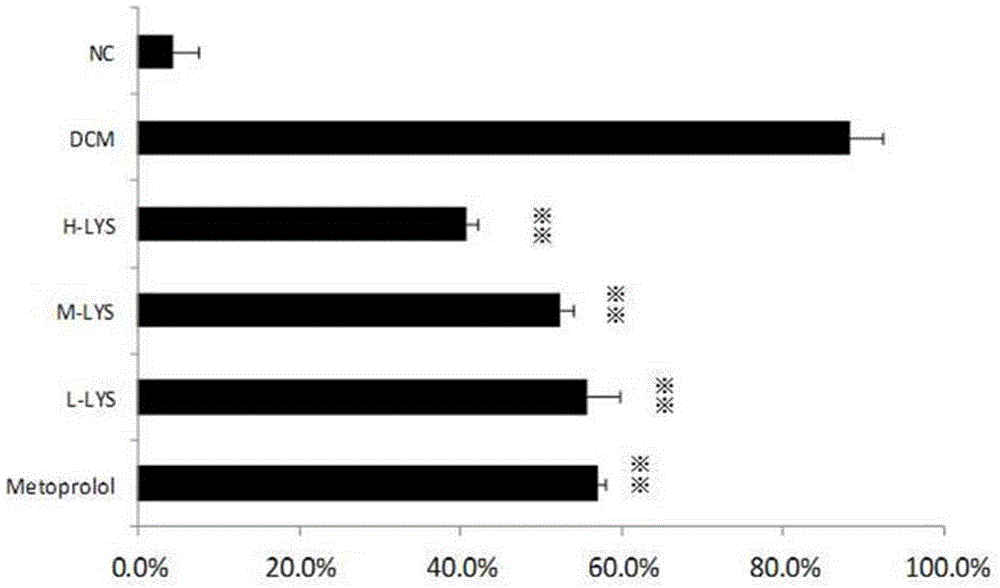
[0030] Take 115 2-week-old Wistar rats, half male and half male, and randomly select 12 rats as the normal control group of the experiment, and feed them with normal rat diet. At the same time, the remaining 103 rats were used as model rats and started to be fed with furazolidone. Rats were fed for 8 weeks.
[0031] After 8 weeks, the dead 4 rat...
example 2
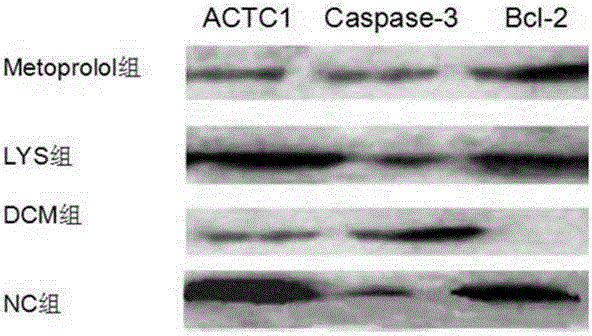
[0063] Example 2: The effect of chlorogenic acid on the expression of ACTC1, caspase-3, Bcl-2 genes and their respective corresponding proteins in cardiomyocytes of dilated cardiomyopathy rat model.
[0064] 1. Experimental materials
[0065] 1.1 Animals
[0066] There are 80 Wistar rats born two weeks old and breast-fed, half male and half male.
[0067] 1.2 Experimental Drugs and Instruments
[0068] Furazolidone, 10% chloral hydrate, PHLIPsonos7500 color ultrasonic detector, PCR instrument, total RNA extraction kit, cDNA first strand synthesis kit, electrophoresis apparatus, gel imager.
[0069] 2. Experimental method
[0070] 2.1 Establishment of rat dilated cardiomyopathy (DCM) model and experimental grouping
[0071] Take 60 2-week-old Wistar rats, half male and half male, randomly select 10 rats as the normal control group of the experiment, and feed them with normal rat diet. At the same time, the remaining 50 rats were used as model rats, and began to be fed with...
Embodiment 3
[0115] Example 3 In vivo pharmacodynamics study on the therapeutic effect of chlorogenic acid on hypertrophic cardiomyopathy
[0116] 1. Experimental materials
[0117] 1.1 Animals
[0118] cT R92Q Transgenic hypertrophic cardiomyopathy (HCM) model C57BL / 6J mice
[0119] 1.2 Experimental Drugs and Instruments
[0120] 10% chloral hydrate, PHLIPsonos7500 color ultrasonic detector, one ten-thousandth electronic balance, high power microscope.
[0121] 2. Experimental method
[0122] 2.1 Grouping of mice
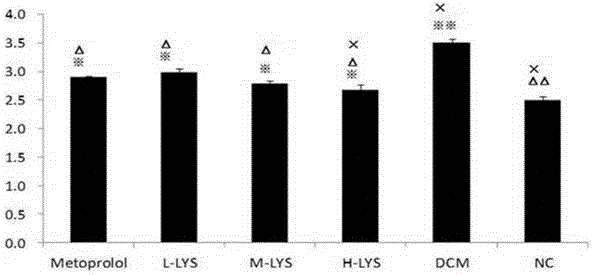
[0123] cTnT R92Q Thirty-two transgenic hypertrophic cardiomyopathy model C57BL / 6J mice were randomly divided into 4 groups with 8 mice in each group. They were respectively set as the model control group (HCM group, n=8), the Metoprolol treatment group (Metoprolol group, n=8), and the chlorogenic acid treatment group (L-LYS group, n=8). At the same time, 8 wild-type C57BL / 6J mice were used as the normal control group (NC group, n=8) for the experiment.
[0124] 2.2 Exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com