Three-dimensional complex cell aggregate model as well as preparation method and application thereof
A three-dimensional composite and aggregate technology, applied in the field of three-dimensional composite cell aggregate model and its preparation, can solve the problems of rare reports of human-derived cadherin protein, achieve the promotion of stem cell proliferation and differentiation function expression, and strengthen specific interaction , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
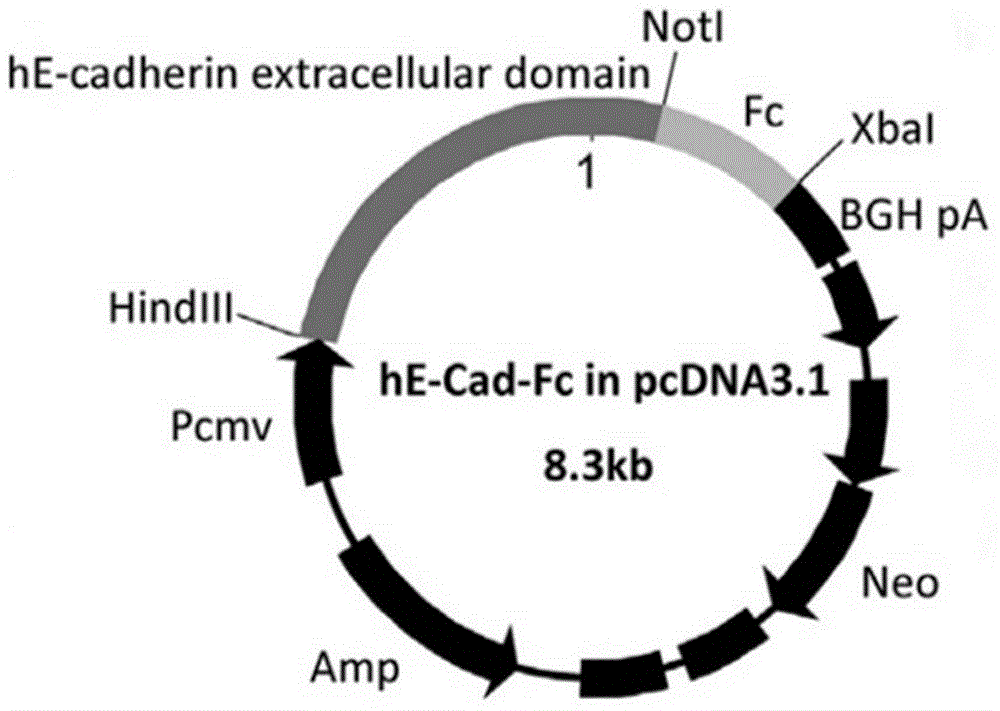
[0057] Embodiment 1 obtains pcDNA3.1-hE-cad-Fc gene plasmid
[0058] Human epithelial cell adhesion factor (hE-cadherin) ectodomain (hE-cad) and human immunoglobulin (IgG 1) Fc fragment (Fc) were fused by gene recombination technology to obtain a plasmid containing the target gene hE-cad and Fc pcDNA3.1-hE-cad-Fc. That is: using the full length of human E-cadherin mRNA (Gene bank: NM 004360.3) as a template, the synthetic primers were used to specifically amplify the hE-cadherin ectodomain fragment. The primer sequences are:
[0059] 5'-CGCAAGCTTATGGGCCCTTG-GAGCCGCAGC-3';
[0060] 5'-TTGCGGCCGCAGGCAGGAATTTGCAATCCTGC-3'.
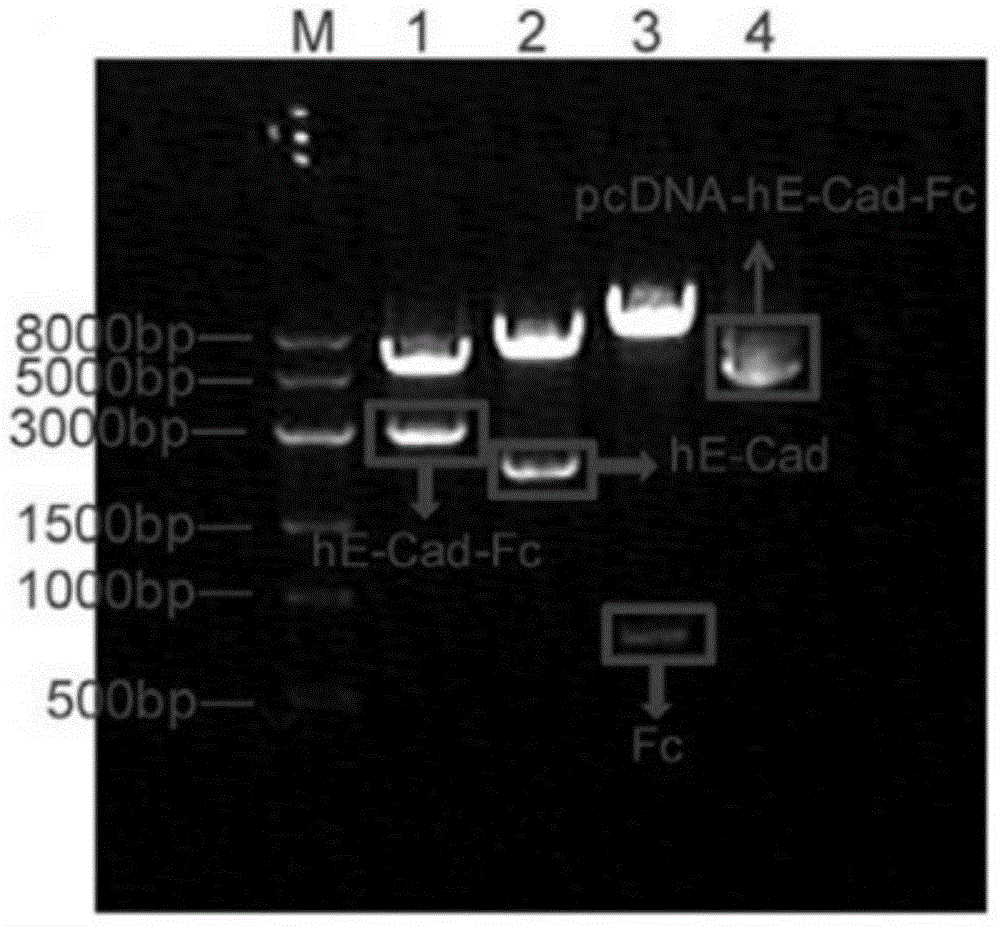
[0061] like figure 1 As shown, the PCR product was analyzed by 1% agarose gel electrophoresis, and the gel-recovered PCR product was double-digested with Hind III and Not I, and inserted between the Hind III and Not I sites of pcDNA3.1-Fc. Plasmid pcDNA3.1-Fc was donated by Professor Toshihiro Akaike of Tokyo Institute of Technology.
[0062] like fig...
Embodiment 2
[0064] Embodiment 2 obtains hE-cad-Fc fusion protein
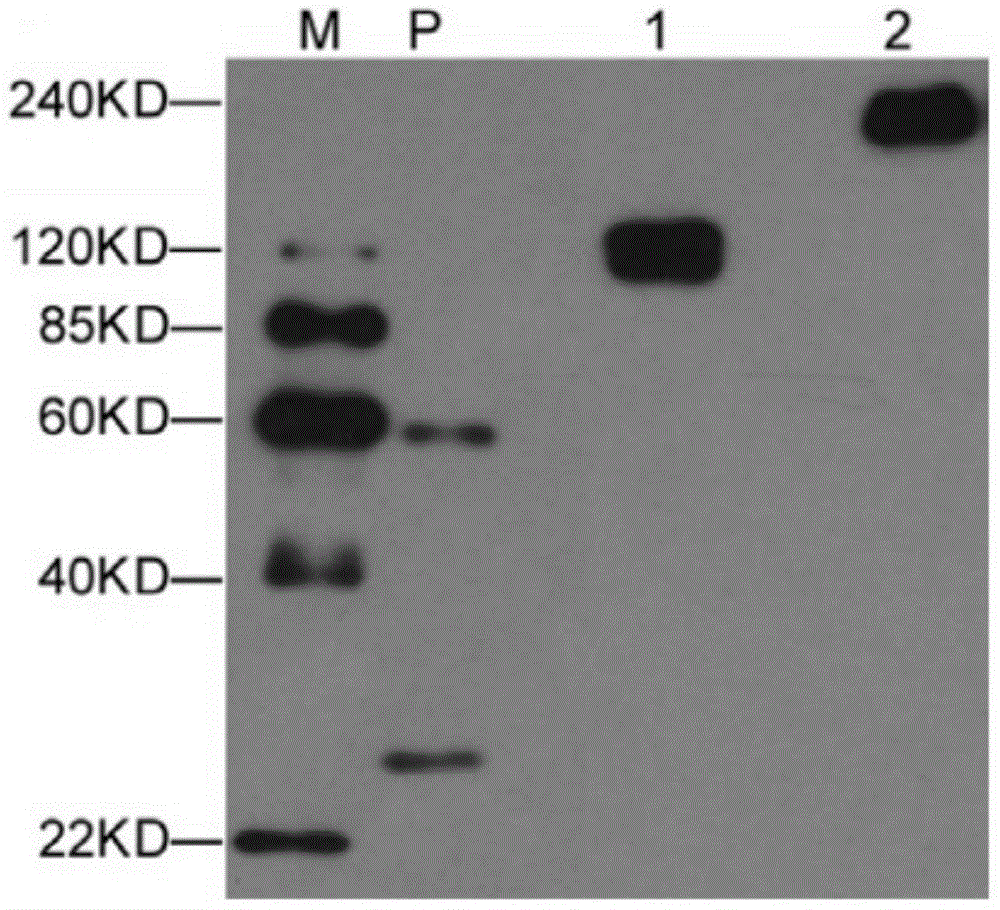
[0065] The plasmid pcDNA3.1-hE-cad-Fc containing the target gene constructed in Example 1 was transfected into 293F cells, cultured in suspension for 72 hours, the cell culture supernatant was collected, and the hE-cad-Fc fusion protein was purified by rProtein AFF column . The purified fusion protein was detected by immunoblotting using an anti-E-cadherin antibody, as image 3 As shown, there are no other non-specific exposure bands in the figure, and the band with a molecular weight of about 120KD is reduced E-cadherin ( image 3 Middle 1), the band at 240KD is non-reduced E-cadherin ( image 3 Medium 2). This result shows that the hE-cad-Fc fusion protein prepared by the present invention has a base sequence as shown in 1 in the sequence table, and the fusion protein can form a stable dimer structure in a non-reduced state.
Embodiment 3
[0066] Example 3 Preparation of hE-cad-Fc fusion protein matrix-modified surface-modified PLGA microspheres
[0067] First, microspheres with a particle size range of 15±5 μm were prepared by chemically synthesizing the polymer PLGA and placed in an EP tube; then the above hE-cad-Fc fusion protein solution was diluted to 10 μg / ml, soaked into the microspheres, and incubated at 37°C for 2 hours; the supernatant was discarded by centrifugation, rinsed with PBS three times to remove the unimmobilized fusion protein hE-cad-Fc, and obtained hE-cad-Fc matrix-modified PLGA microspheres, which were named hE-cad- Fc-PLGA.
[0068] The invention detects the effect of the protein concentration of the hE-cad-Fc solution on the surface modification of the matrix of PLGA microspheres by an Elisa method. The hE-cad-Fc substrate method is the same as above. Namely: after obtaining hE-cad-Fc-PLGA, add 1% BSA to block at room temperature for 1 hour, add anti-E-cadherin antibody and incubate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com