Arabinogalactan of flowers of panax notoginseng (burK.)F.H.Chen, and preparation method and use thereof
A technology of arabinose and Panax notoginseng is applied in the field of preparing antitumor drugs, which can solve the problems of low toxicity and side effects, high mortality of pancreatic cancer, and difficulty in early clinical diagnosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
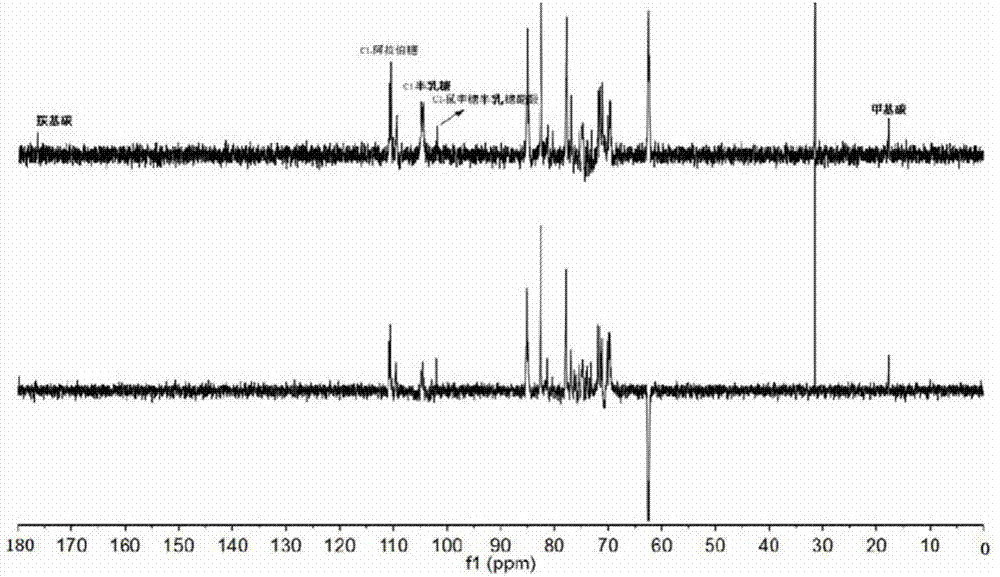
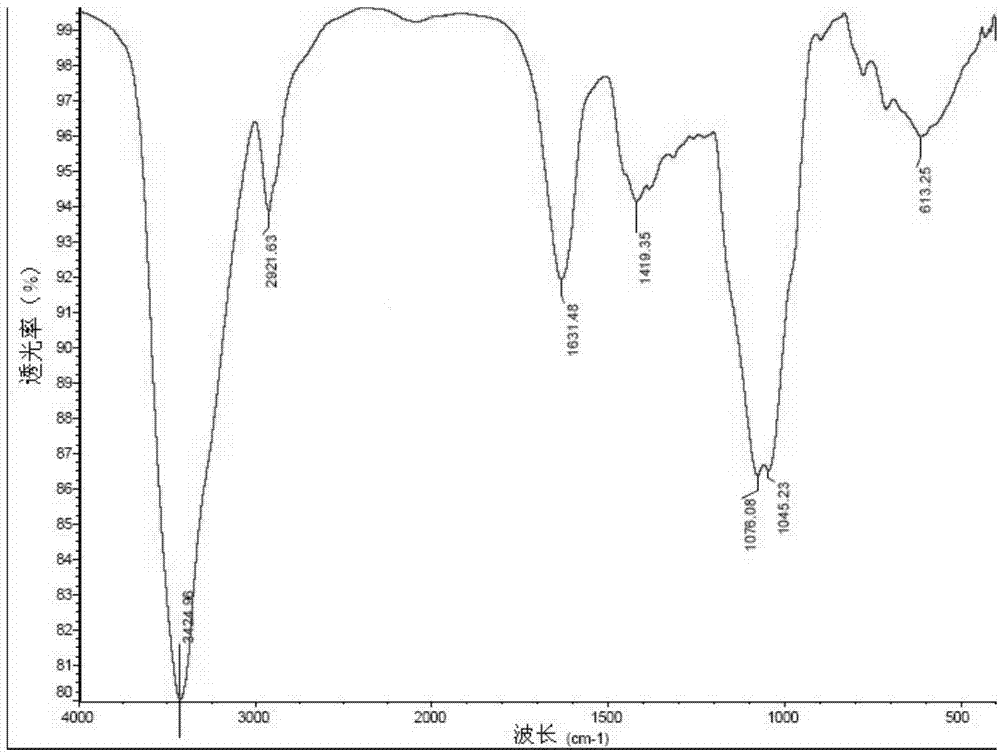
[0028] Example 1: Preparation of Arabinogalactan RN1
[0029] a. Polysaccharide extraction:
[0030] Dried notoginseng flowers were degreased with 95% ethanol for one week, and then dried naturally at room temperature. 1000 g of the dried notoginseng flower was extracted 5 times with 20 liters of boiling water (deionized water), 6 hours each time. Sulfuric acid-phenol detected no obvious reaction, filtered, combined the extracts each time, heated and concentrated to 2 liters, added three times the volume of 6 liters of 95% ethanol under stirring, stood overnight, poured the supernatant, and centrifuged After separation, the obtained precipitate was washed with 2 times the volume of absolute ethanol, centrifuged, and the precipitate was vacuum-dried at 40° C. to obtain 100 g of crude polysaccharide from Panax notoginseng flower extracted by water.
[0031] b. Polysaccharide purification:
[0032] Take 10 g of the Panax notoginseng crude polysaccharide prepared above, dissolv...
Embodiment 2
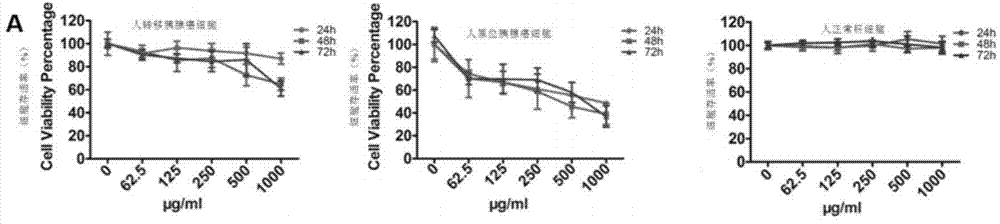
[0040] Example 2: Arabinogalactan RN1 inhibits tumor activity of pancreatic cancer
[0041] MTT (tetrazolium salt method) experiment
[0042] In the experiment, human pancreatic cancer cell lines BxPC-3 and AsPC-1 and human normal liver cell line LO2 (Cell Bank of Typical Culture Collection Committee of Chinese Academy of Sciences, Cell Resource Center of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences) were used to culture in 10% fetal bovine serum (Gibco), 100 U / mL penicillin and 100 U / mL streptomycin in DMEM medium (HyClone). Cells at 37°C with 5% CO 2 cultured in an incubator.
[0043] BxPC-3, AsPC-1, LO2 cells in logarithmic growth phase 5×10 3 were inoculated in a 96-well plate, discarded the supernatant after incubation in an incubator for 24 hours, and added 100 μL of a culture medium solution containing uniform polysaccharide RN1 (prepared in Example 1) to make the final concentration 62.5, 125, 250, 500 and 1000 μg / mL , each concentration h...
Embodiment 3
[0046] Example 3: Polysaccharide RN1 inhibits neovascularization and its migration
[0047] In vitro angiogenesis assay
[0048] In the experiment, human epidermal vascular cells HMEC-1 (founded by Emory University in the United States and preserved in the Shanghai Institute of Materia Medica, Chinese Academy of Sciences) were cultured in a medium containing 15% fetal bovine serum (Gibco Company), 2mM L-glutamine, 10ng / mL EGF, 100U / mL penicillin and 100U / mL streptomycin in MCDB131 medium (Gibco). Cells at 37°C with 5% CO 2 cultured in an incubator.
[0049] Add 50 μL of thawed Matrigel at 4°C to a 96-well plate pre-cooled at 4°C, solidify at 37°C for 30 minutes, and then add 4.5×10 HMEC-1 cells containing 0, 0.5 mg / mL and 1 mg / mL RN1 4 were cultured for 12 h in the cell culture incubator. The results were recorded with an inverted microscope at a magnification of 40 times, and the average length of the lumen in the field of view was counted using Image J software.
[0050...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com