Recycling ammonia desulphurization process
An ammonia-based desulfurization and recycling technology, applied in the field of flue gas desulfurization, can solve the problems of high cost of manganese carbonate and low oxidation efficiency, achieve high product quality, simple process operation, and meet the requirements of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
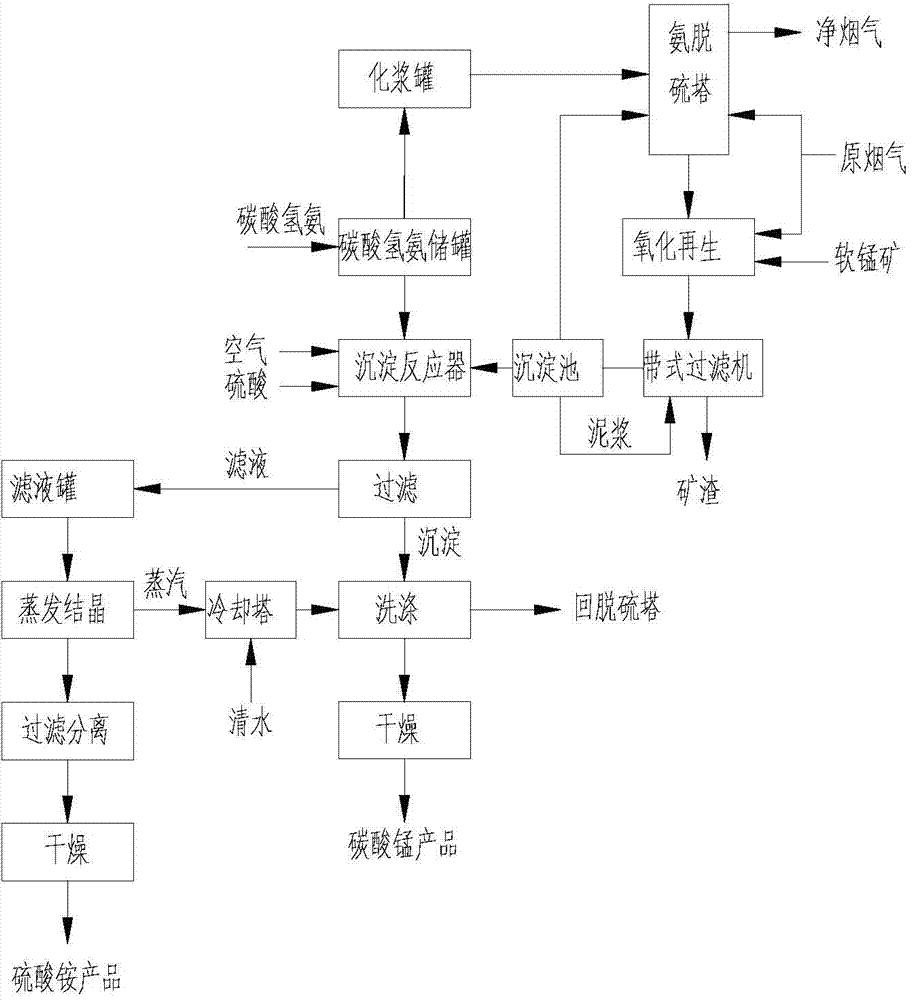
[0043] A. Desulfurization: Ammonium bicarbonate, part of ammonium sulfate solution, and a certain proportion of process water are configured to form an ammonium bicarbonate solution with a concentration of 20% ammonium bicarbonate, which is input into the ammonium absorption tower through the slurry pump and is in reverse contact with the flue gas. SO 2 The concentration is 2100mg / Nm 3 The flow velocity of the flue gas passing through the absorption tower is 5m / s, and the liquid-gas ratio in the tower is 10L / m 3 , ammonium bicarbonate and SO in flue gas 2 A reaction occurs and ammonium bisulfite is formed. After desulfurization, SO 2 The removal efficiency is 97%, SO in the net flue gas 2 The concentration is 65mg / Nm 3 .
[0044] B. Oxidation regeneration: the desulfurized slurry is transported to the oxidation regeneration reactor, where the ammonium bisulfite is replaced by MnO in pyrolusite (manganese content 25%, 220 mesh) 2 For oxidation regeneration, the blower blo...
Embodiment 2
[0048] A. Desulfurization: Ammonium bicarbonate, part of ammonium sulfate solution, and a certain proportion of process water are configured to form an ammonium bicarbonate solution with a concentration of 26% ammonium bicarbonate, which is input into the ammonium absorption tower through the slurry pump and is in reverse contact with the flue gas. SO 2 The concentration is 2400mg / Nm 3 The flow velocity of the flue gas passing through the absorption tower is 3.4m / s, and the liquid-gas ratio in the tower is 10L / m 3 , ammonium bicarbonate and SO in flue gas 2 A reaction occurs and ammonium bisulfite is formed. After desulfurization, SO 2 The removal efficiency is 97%, SO in the net flue gas 2 The concentration is 72mg / Nm 3 .
[0049] B. Oxidation regeneration: the desulfurized slurry is transported to the oxidation regeneration reactor, where the ammonium bisulfite is replaced by MnO in pyrolusite (30% manganese content, 220 mesh) 2 Oxidation regeneration, the blower blows...
Embodiment 3
[0053] A. Desulfurization: ammonium bicarbonate and the precipitated liquid in Step C of Example 2 and a certain proportion of process water are configured to form an ammonium bicarbonate solution with a concentration of 18% ammonium bicarbonate, which is input into the ammonium absorption tower through the slurry pump and is in reverse contact with the flue gas. , SO 2 The concentration is 2000mg / Nm 3 The flow velocity of the flue gas passing through the absorption tower is 5m / s, and the liquid-gas ratio in the tower is 10L / m 3 , ammonium bicarbonate and SO in flue gas 2 A reaction occurs and ammonium bisulfite is formed. After desulfurization, SO 2 The removal efficiency is 97%, SO in the net flue gas 2 The concentration is 60mg / Nm 3 .
[0054] B. Oxidation regeneration: the desulfurized slurry is transported to the oxidation regeneration reactor, where the ammonium bisulfite is replaced by MnO in pyrolusite (30% manganese content, 220 mesh) 2 For oxidation regenerati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com