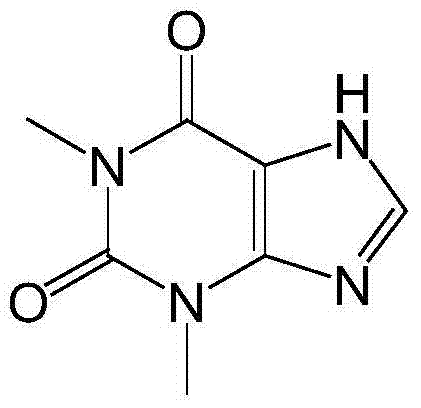
Synthetic method of high-purity high-yield theophylline
A synthesis method and a high-yield technology are applied in the field of high-purity and high-yield theophylline synthesis, can solve the problems of low purity of theophylline, low theophylline yield, complicated processes, etc., and achieve the safety and environmental protection of reagents and the preparation process. Simple, easy-to-operate preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
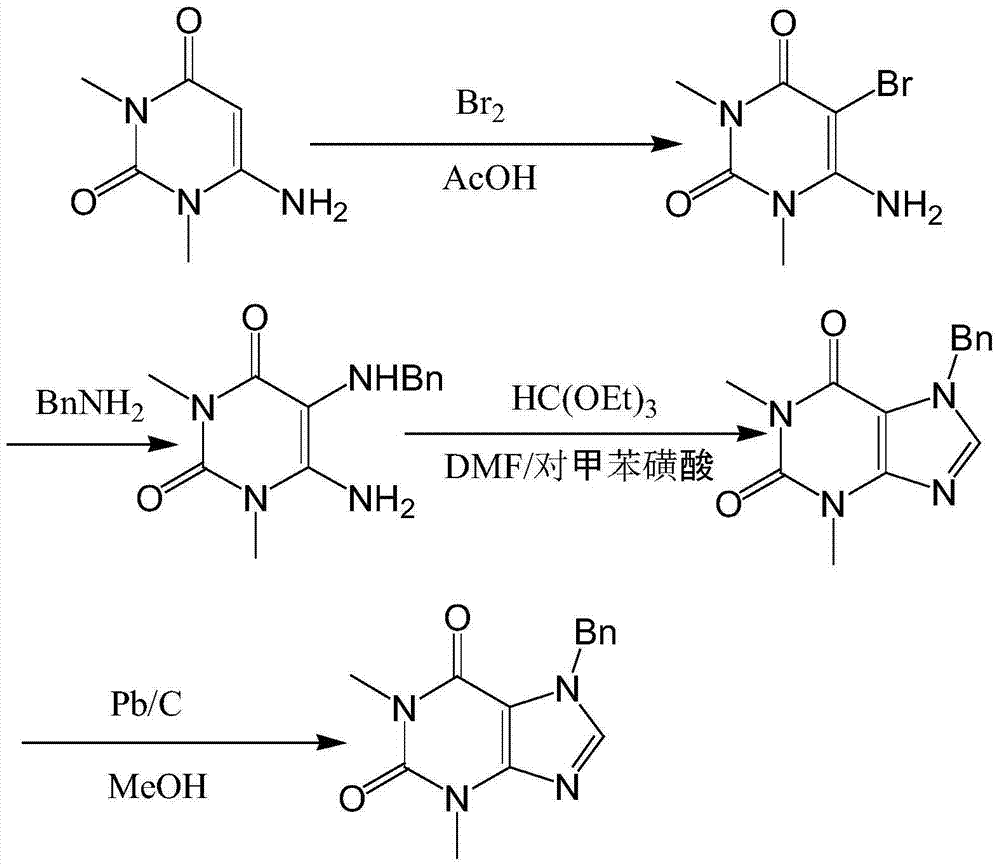
[0030] The method for synthesizing theophylline with high purity and high yield provided by the present invention includes the following steps:
[0031] S1. Put sodium acetate trihydrate, 6-amino-1,3-dimethyluracil, and anhydrous acetic acid in the first reaction vessel, heat up to 124-127°C, keep for 28-31min, and cool down to 79-82 ℃, then add the mixed solution, reduce to room temperature, stir for 47-50h, after filtration, wash the filter cake with 98wt% glacial acetic acid, and then dry to obtain 6-amino-5-bromo-1,3-dimethyluracil , Where the mixed solution is made by mixing liquid bromine and anhydrous acetic acid;
[0032] S2. Add 6-amino-5-bromo-1,3-dimethyluracil and benzylamine into the second reaction vessel, raise the temperature to 98-100°C, keep the temperature for 1.9-2.2h, and keep the temperature up and down during the heat preservation process. Stop stirring, then lower to room temperature, add ice water until no solids continue to precipitate, wash the filter ca...
Embodiment 1
[0037] The method for synthesizing theophylline with high purity and high yield provided by the present invention includes the following steps:
[0038] S1. Put sodium acetate trihydrate, 6-amino-1,3-dimethyluracil, and anhydrous acetic acid in the first reaction vessel, heat up to 125°C, keep for 30min, cool down to 80°C, and then add the mixed solution , Cooled to room temperature, stirred for 48h, filtered, washed the filter cake with 98wt% glacial acetic acid, and then dried to obtain 6-amino-5-bromo-1,3-dimethyluracil, in which the mixed solution is composed of liquid bromine and Mixed with anhydrous acetic acid;
[0039] S2. Add 6-amino-5-bromo-1,3-dimethyluracil and benzylamine into the second reaction vessel, raise the temperature to 100℃, keep it for 2h, keep stirring during the heating process and the heat preservation process, and then reduce After reaching room temperature, add ice water until no solids continue to precipitate out, wash the filter cake with ice water a...
Embodiment 2
[0043] The method for synthesizing theophylline with high purity and high yield provided by the present invention includes the following steps:
[0044] S1. Molybdenum Put 1.4 parts of sodium acetate trihydrate, 2 parts of 6-amino-1,3-dimethyluracil, and anhydrous acetic acid in the first reaction vessel, raise the temperature to 124°C, keep the temperature for 31min, and cool it down to At 79°C, add the mixed solution, reduce to room temperature, stir for 50h, after filtration, wash the filter cake with 98wt% glacial acetic acid, and then dry to obtain 6-amino-5-bromo-1,3-dimethyluracil. The mixed solution is made by mixing 1 part of liquid bromine and 4.3 parts of anhydrous acetic acid;
[0045] S2. Add 6-amino-5-bromo-1,3-dimethyluracil and benzylamine into the second reaction vessel, increase the temperature to 98°C, keep the temperature for 2.2h, keep stirring during the heating process and the insulation process, and then After cooling down to room temperature, add ice water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com