Preparation method and application of insulin detemir crystal
A technology of insulin detemir and crystallization, which is applied in the field of preparation of insulin detemir crystals, can solve problems such as the unfavorable structure stability of insulin detemir, and achieve the effects of overcoming crystallization difficulties, small crystal sedimentation volume, and short freeze-drying time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
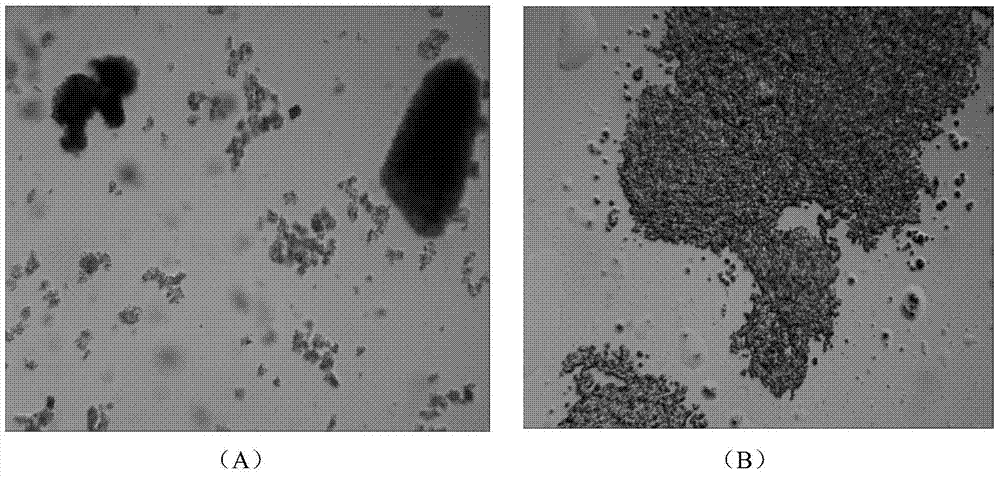
Embodiment 1
[0037] Refer to Example 1 and Example 2 in the Chinese patent CN201110118026.2 titled "Method for preparing insulin glargine crystals" to prepare insulin detemir crystals. The ingredients and concentrations are as follows: Experimental group A is insulin detemir 3.5g / L, acetonitrile 20% (v / v), acetic acid 0.7M, phenol 0.4% (w / v), zinc chloride 120mg / L, sodium acetate 0.5M; experimental group B was insulin detemir 3g / L, ethanol 15% (v / v), acetic acid 0.5M, zinc chloride 90mg / L, phenol 0.2% (w / v), sodium chloride 0.3M. The preparation process is as follows: Weigh two parts of insulin detemir, A1: 0.175g, B1: 0.15g, add 20ml of water respectively to suspend for use; prepare saline solution a1: 10ml of acetonitrile, 2ml of acetic acid, 6% (w / v) 3.33ml of phenol aqueous solution, 3.4g of sodium acetate and 55μl of 0.5M zinc acetate solution, add water to dissolve and dilute to 25ml; prepare salt solution b1: 7.5ml of ethanol, 1.43ml of acetic acid, 1.68ml of 6% (w / v) phenol aqueou...
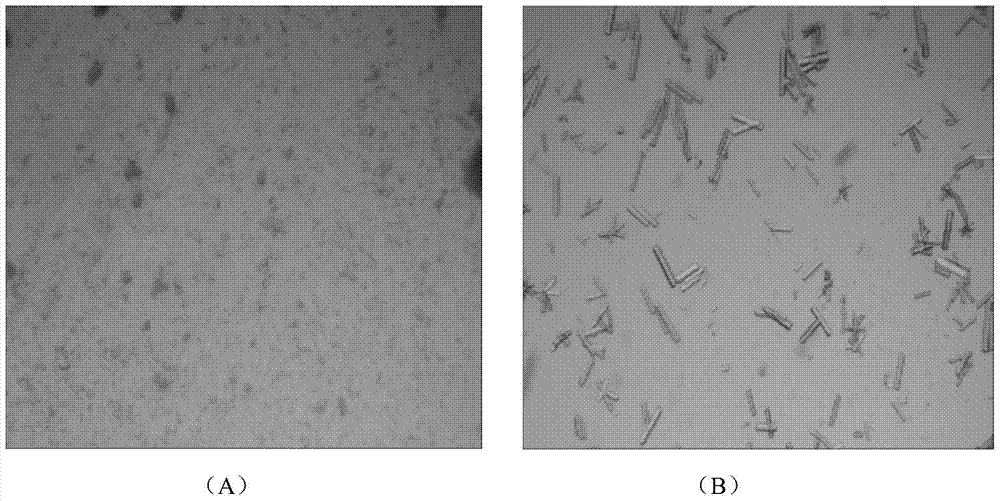
Embodiment 2
[0039] Prepare insulin detemir crystal liquid, each component concentration is: experimental group A is insulin detemir 2g / L, acetonitrile 7.5% (v / v), ammonium acetate 0.1M, sodium chloride 0.2M, phenol 0.2% (w / v) The molar ratio of zinc ions to insulin detemir was 2:1; for experimental group B, 0.1M trisodium citrate dihydrate was added on the basis of experimental group A.
[0040] The preparation process is as follows: Weigh 0.2g insulin detemir, add 50ml of acetonitrile aqueous solution with a concentration of 15% (v / v), adjust the pH value to 8.57 with 2M sodium hydroxide solution, dissolve completely, and set aside; prepare salt solution a2: acetic acid Ammonium 0.39g, sodium chloride 0.58g, 6% (w / v) phenol aqueous solution 1.68ml, add water to dissolve and set the volume to 25ml, 2M sodium hydroxide to adjust the pH value to 8.86; prepare salt solution b2: in solution a2 On top of that, 1.47 g of trisodium citrate dihydrate was added. Take two 4g / L insulin detemir sol...
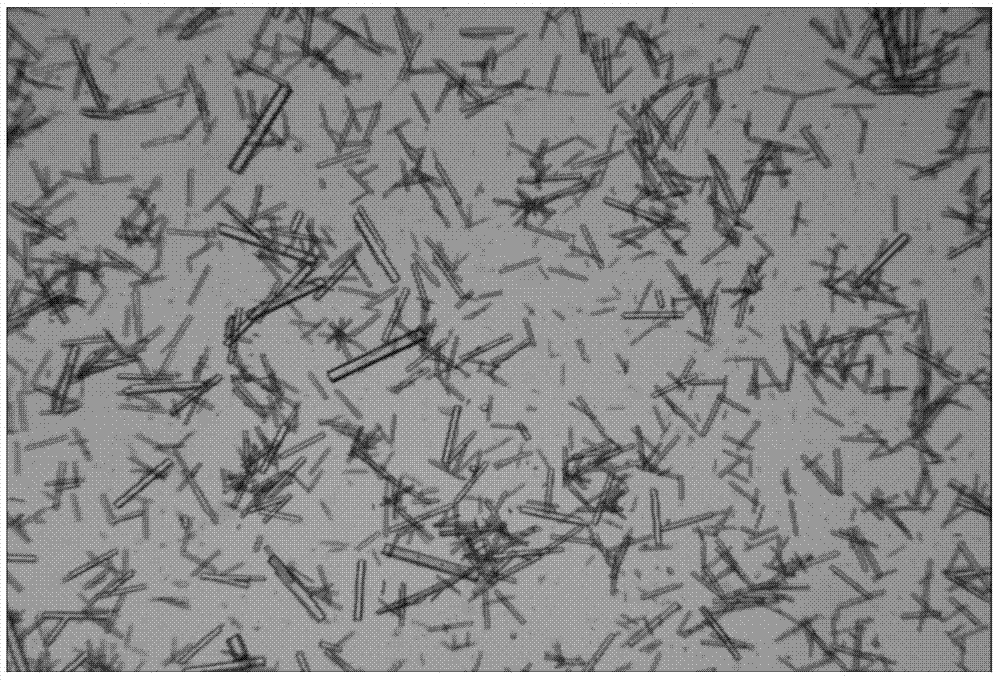
Embodiment 3
[0042] Prepare insulin detemir crystal liquid, the concentration of each component is: insulin detemir 1g / L, acetonitrile concentration 5% (v / v), trisodium citrate 0.2M, trishydrochloride 0.4M, phenol 0.05% (w / v), the molar ratio of zinc ions to insulin detemir is 7:1.
[0043] The preparation process is as follows: Weigh 0.05 g of insulin detemir, add 25 ml of acetonitrile aqueous solution with a concentration of 10% (v / v), adjust the pH value to 8.64 with 2M sodium hydroxide solution, dissolve completely, and set aside; prepare salt solution: dihydrate 2.94 g of trisodium citrate, 3.15 g of trishydroxymethylaminomethane hydrochloride, and 420 μl of aqueous phenol solution with a concentration of 6% (w / v), were dissolved in water and adjusted to 25 ml, and the pH value was adjusted to 2M sodium hydroxide solution. 8.78, and added to 25ml of insulin detemir solution, mixed; finally added 118μl of 0.5M zinc acetate solution, 2M hydrochloric acid to adjust the pH value to 8.21; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com